Biophysical markers of the peripheral vasoconstriction response to pain in sickle cell disease
- PMID: 28542469
- PMCID: PMC5443571
- DOI: 10.1371/journal.pone.0178353
Biophysical markers of the peripheral vasoconstriction response to pain in sickle cell disease
Abstract
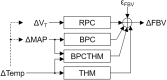
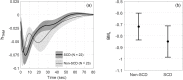
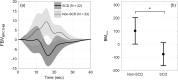
Painful vaso-occlusive crisis (VOC), a complication of sickle cell disease (SCD), occurs when sickled red blood cells obstruct flow in the microvasculature. We postulated that exaggerated sympathetically mediated vasoconstriction, endothelial dysfunction and the synergistic interaction between these two factors act together to reduce microvascular flow, promoting regional vaso-occlusions, setting the stage for VOC. We previously found that SCD subjects had stronger vasoconstriction response to pulses of heat-induced pain compared to controls but the relative degrees to which autonomic dysregulation, peripheral vascular dysfunction and their interaction are present in SCD remain unknown. In the present study, we employed a mathematical model to decompose the total vasoconstriction response to pain into: 1) the neurogenic component, 2) the vascular response to blood pressure, 3) respiratory coupling and 4) neurogenic-vascular interaction. The model allowed us to quantify the contribution of each component to the total vasoconstriction response. The most salient features of the components were extracted to represent biophysical markers of autonomic and vascular impairment in SCD and controls. These markers provide a means of phenotyping severity of disease in sickle-cell anemia that is based more on underlying physiology than on genotype. The marker of the vascular component (BMv) showed stronger contribution to vasoconstriction in SCD than controls (p = 0.0409), suggesting a dominant myogenic response in the SCD subjects as a consequence of endothelial dysfunction. The marker of neurogenic-vascular interaction (BMn-v) revealed that the interaction reinforced vasoconstriction in SCD but produced vasodilatory response in controls (p = 0.0167). This marked difference in BMn-v suggests that it is the most sensitive marker for quantifying combined alterations in autonomic and vascular function in SCD in response to heat-induced pain.
Conflict of interest statement
Figures



Similar articles
-
Mental stress causes vasoconstriction in subjects with sickle cell disease and in normal controls.Haematologica. 2020 Jan;105(1):83-90. doi: 10.3324/haematol.2018.211391. Epub 2019 Apr 11. Haematologica. 2020. PMID: 30975906 Free PMC article.
-
Individuals with sickle cell disease have a significantly greater vasoconstriction response to thermal pain than controls and have significant vasoconstriction in response to anticipation of pain.Am J Hematol. 2017 Nov;92(11):1137-1145. doi: 10.1002/ajh.24858. Epub 2017 Aug 17. Am J Hematol. 2017. PMID: 28707371 Free PMC article.
-
Progressive vasoconstriction with sequential thermal stimulation indicates vascular dysautonomia in sickle cell disease.Blood. 2020 Sep 3;136(10):1191-1200. doi: 10.1182/blood.2020005045. Blood. 2020. PMID: 32518948 Free PMC article.
-
Autonomic nervous system dysfunction: implication in sickle cell disease.C R Biol. 2013 Mar;336(3):142-7. doi: 10.1016/j.crvi.2012.09.003. Epub 2012 Oct 16. C R Biol. 2013. PMID: 23643396 Review.
-
Vaso-Occlusion in Sickle Cell Disease: Is Autonomic Dysregulation of the Microvasculature the Trigger?J Clin Med. 2019 Oct 15;8(10):1690. doi: 10.3390/jcm8101690. J Clin Med. 2019. PMID: 31618931 Free PMC article. Review.
Cited by
-
Sickle Cell Disease Subjects Have a Distinct Abnormal Autonomic Phenotype Characterized by Peripheral Vasoconstriction With Blunted Cardiac Response to Head-Up Tilt.Front Physiol. 2019 Apr 11;10:381. doi: 10.3389/fphys.2019.00381. eCollection 2019. Front Physiol. 2019. PMID: 31031633 Free PMC article.
-
Mental stress causes vasoconstriction in subjects with sickle cell disease and in normal controls.Haematologica. 2020 Jan;105(1):83-90. doi: 10.3324/haematol.2018.211391. Epub 2019 Apr 11. Haematologica. 2020. PMID: 30975906 Free PMC article.
-
Mathematical modeling of SCD: a literature review.J Sick Cell Dis. 2025 Apr 16;2(1):yoaf015. doi: 10.1093/jscdis/yoaf015. eCollection 2025. J Sick Cell Dis. 2025. PMID: 40444258 Free PMC article. Review.
-
A novel cross-correlation methodology for assessing biophysical responses associated with pain.J Pain Res. 2018 Oct 5;11:2207-2219. doi: 10.2147/JPR.S142582. eCollection 2018. J Pain Res. 2018. PMID: 30323655 Free PMC article.
-
Functional near-infrared spectroscopy-based prefrontal cortex oxygenation during working memory tasks in sickle cell disease.Neurophotonics. 2023 Oct;10(4):045004. doi: 10.1117/1.NPh.10.4.045004. Epub 2023 Oct 17. Neurophotonics. 2023. PMID: 37854507 Free PMC article.
References
-
- Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010;376(9757):2018–31. doi: 10.1016/S0140-6736(10)61029-X - DOI - PubMed
-
- Eaton WA, Hofrichter J, Ross PD. Editorial: Delay time of gelation: a possible determinant of clinical severity in sickle cell disease. Blood. 1976. April;47(4):621–7. - PubMed
-
- Sangkatumvong S, Khoo MCK, Kato R, Detterich JA, Bush A, Keens TG, et al. Peripheral vasoconstriction and abnormal parasympathetic response to sighs and transient hypoxia in sickle cell disease. Am J Respir Crit Care Med. 2011;184:474–81. doi: 10.1164/rccm.201103-0537OC - DOI - PMC - PubMed
-
- Schobel HP, Ringkamp M, Behrmann A, Forster C, Schmieder RE, Handwerker HO. Hemodynamic and sympathetic nerve responses to painful stimuli in normotensive and borderline hypertensive subjects. Pain. 1996. August;66(2):117–24. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

