D-helix influences dimerization of the ATP-binding cassette (ABC) transporter associated with antigen processing 1 (TAP1) nucleotide-binding domain
- PMID: 28542489
- PMCID: PMC5441636
- DOI: 10.1371/journal.pone.0178238
D-helix influences dimerization of the ATP-binding cassette (ABC) transporter associated with antigen processing 1 (TAP1) nucleotide-binding domain
Abstract
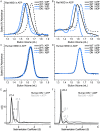
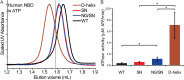
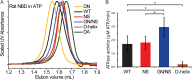
ATP-binding cassette (ABC) transporters form a large family of transmembrane importers and exporters. Using two nucleotide-binding domains (NBDs), which form a canonical ATP-sandwich dimer at some point within the transport cycle, the transporters harness the energy from ATP binding and hydrolysis to drive substrate transport. However the structural elements that enable and tune the dimerization propensity of the NBDs have not been fully elucidated. Here we compared the biochemical properties of the NBDs of human and rat TAP1, a subunit of the heterodimeric transporter associated with antigen processing (TAP). The isolated human TAP1 NBD was monomeric in solution, in contrast to the previously observed ATP-mediated homodimerization of the isolated rat TAP1 NBD. Using a series of human-rat chimeric constructs, we identified the D-helix, an α-helix N-terminal to the conserved D-loop motif, as an important determinant of NBD dimerization. The ATPase activity of our panel of TAP1 NBD constructs largely correlated with dimerization ability, indicating that the observed dimerization uses the canonical ATP-sandwich interface. The N-terminus of the D-helix from one protomer interacts with the ATP-binding Walker A motif of the second protomer at the ATP-sandwich interface. However, our mutational analysis indicated that residues farther from the interface, within the second and third turn of the D-helix, also influence dimerization. Overall, our data suggest that although the D-helix sequence is not conserved in ABC transporters, its precise positioning within the NBD structure has a critical role in NBD dimerization.
Conflict of interest statement
Figures


Similar articles
-
Functional non-equivalence of ATP-binding cassette signature motifs in the transporter associated with antigen processing (TAP).J Biol Chem. 2004 Oct 29;279(44):46073-81. doi: 10.1074/jbc.M404042200. Epub 2004 Aug 17. J Biol Chem. 2004. PMID: 15322097
-
The distinct nucleotide binding states of the transporter associated with antigen processing (TAP) are regulated by the nonhomologous C-terminal tails of TAP1 and TAP2.Eur J Biochem. 2003 Nov;270(22):4531-46. doi: 10.1046/j.1432-1033.2003.03848.x. Eur J Biochem. 2003. PMID: 14622282
-
ATP-induced conformational changes of nucleotide-binding domains in an ABC transporter. Importance of the water-mediated entropic force.J Phys Chem B. 2014 Nov 6;118(44):12612-20. doi: 10.1021/jp507930e. Epub 2014 Oct 24. J Phys Chem B. 2014. PMID: 25302667
-
What monomeric nucleotide binding domains can teach us about dimeric ABC proteins.FEBS Lett. 2020 Dec;594(23):3857-3875. doi: 10.1002/1873-3468.13921. Epub 2020 Sep 17. FEBS Lett. 2020. PMID: 32880928 Review.
-
Structurally diverse C-terminal accessory domains in type I ABC importers reveal distinct regulatory mechanisms.Structure. 2025 May 1;33(5):843-857. doi: 10.1016/j.str.2025.02.014. Epub 2025 Mar 24. Structure. 2025. PMID: 40132581 Review.
Cited by
-
The C-terminal α-helix is crucial for the activity of the bacterial ABC transporter BmrA.J Biol Chem. 2025 Feb;301(2):108098. doi: 10.1016/j.jbc.2024.108098. Epub 2024 Dec 18. J Biol Chem. 2025. PMID: 39706270 Free PMC article.
-
Cryo-EM analyses unveil details of mechanism and targocil-II mediated inhibition of S. aureus WTA transporter TarGH.Nat Commun. 2025 Apr 4;16(1):3224. doi: 10.1038/s41467-025-58202-w. Nat Commun. 2025. PMID: 40185711 Free PMC article.
-
Nsp1α of Porcine Reproductive and Respiratory Syndrome Virus Strain BB0907 Impairs the Function of Monocyte-Derived Dendritic Cells via the Release of Soluble CD83.J Virol. 2018 Jul 17;92(15):e00366-18. doi: 10.1128/JVI.00366-18. Print 2018 Aug 1. J Virol. 2018. PMID: 29793955 Free PMC article.
-
Combining Mutations That Inhibit Two Distinct Steps of the ATP Hydrolysis Cycle Restores Wild-Type Function in the Lipopolysaccharide Transporter and Shows that ATP Binding Triggers Transport.mBio. 2019 Aug 20;10(4):e01931-19. doi: 10.1128/mBio.01931-19. mBio. 2019. PMID: 31431556 Free PMC article.
-
Targeting Nucleotide Binding Domain of Multidrug Resistance-associated Protein-1 (MRP1) for the Reversal of Multi Drug Resistance in Cancer.Sci Rep. 2018 Aug 10;8(1):11973. doi: 10.1038/s41598-018-30420-x. Sci Rep. 2018. PMID: 30097643 Free PMC article.
References
-
- Dean M, Hamon Y, Chimini G. The human ATP-binding cassette (ABC) transporter superfamily. J Lipid Res. 2001;42(7):1007–17. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

