Zinc blocks SOS-induced antibiotic resistance via inhibition of RecA in Escherichia coli
- PMID: 28542496
- PMCID: PMC5440055
- DOI: 10.1371/journal.pone.0178303
Zinc blocks SOS-induced antibiotic resistance via inhibition of RecA in Escherichia coli
Abstract
Zinc inhibits the virulence of diarrheagenic E. coli by inducing the envelope stress response and inhibiting the SOS response. The SOS response is triggered by damage to bacterial DNA. In Shiga-toxigenic E. coli, the SOS response strongly induces the production of Shiga toxins (Stx) and of the bacteriophages that encode the Stx genes. In E. coli, induction of the SOS response is accompanied by a higher mutation rate, called the mutator response, caused by a shift to error-prone DNA polymerases when DNA damage is too severe to be repaired by canonical DNA polymerases. Since zinc inhibited the other aspects of the SOS response, we hypothesized that zinc would also inhibit the mutator response, also known as hypermutation. We explored various different experimental paradigms to induce hypermutation triggered by the SOS response, and found that hypermutation was induced not just by classical inducers such as mitomycin C and the quinolone antibiotics, but also by antiviral drugs such as zidovudine and anti-cancer drugs such as 5-fluorouracil, 6-mercaptopurine, and azacytidine. Zinc salts inhibited the SOS response and the hypermutator phenomenon in E. coli as well as in Klebsiella pneumoniae, and was more effective in inhibiting the SOS response than other metals. We then attempted to determine the mechanism by which zinc, applied externally in the medium, inhibits hypermutation. Our results show that zinc interferes with the actions of RecA, and protects LexA from RecA-mediated cleavage, an early step in initiation of the SOS response. The SOS response may play a role in the development of antibiotic resistance and the effect of zinc suggests ways to prevent it.
Conflict of interest statement
Figures
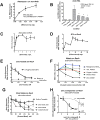
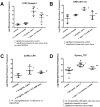
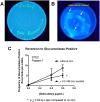
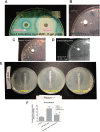

References
-
- Zhang X, McDaniel A, Wolf L, Keusch G, Waldor M, Acheson D. Quinolone antibiotics induce Shiga toxin-encoding bacteriophages, toxin production, and death in mice. J Infectious Dis. 2000;181:664–70. - PubMed
-
- Goodman MF. Error-prone repair DNA polymerases in prokaryotes and eukaryotes. Annual review of biochemistry. 2002;71(1):17–50. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

