Varicella zoster virus glycoprotein C increases chemokine-mediated leukocyte migration
- PMID: 28542541
- PMCID: PMC5444840
- DOI: 10.1371/journal.ppat.1006346
Varicella zoster virus glycoprotein C increases chemokine-mediated leukocyte migration
Abstract
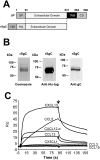
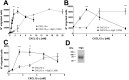
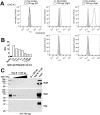
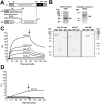
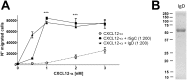
Varicella zoster virus (VZV) is a highly prevalent human pathogen that establishes latency in neurons of the peripheral nervous system. Primary infection causes varicella whereas reactivation results in zoster, which is often followed by chronic pain in adults. Following infection of epithelial cells in the respiratory tract, VZV spreads within the host by hijacking leukocytes, including T cells, in the tonsils and other regional lymph nodes, and modifying their activity. In spite of its importance in pathogenesis, the mechanism of dissemination remains poorly understood. Here we addressed the influence of VZV on leukocyte migration and found that the purified recombinant soluble ectodomain of VZV glycoprotein C (rSgC) binds chemokines with high affinity. Functional experiments show that VZV rSgC potentiates chemokine activity, enhancing the migration of monocyte and T cell lines and, most importantly, human tonsillar leukocytes at low chemokine concentrations. Binding and potentiation of chemokine activity occurs through the C-terminal part of gC ectodomain, containing predicted immunoglobulin-like domains. The mechanism of action of VZV rSgC requires interaction with the chemokine and signalling through the chemokine receptor. Finally, we show that VZV viral particles enhance chemokine-dependent T cell migration and that gC is partially required for this activity. We propose that VZV gC activity facilitates the recruitment and subsequent infection of leukocytes and thereby enhances VZV systemic dissemination in humans.
Conflict of interest statement
AEIP's affiliation is NovImmune, Geneva, Switzerland. The authors have declared that no competing interests exist.
Figures
Similar articles
-
Dysregulated Glycoprotein B-Mediated Cell-Cell Fusion Disrupts Varicella-Zoster Virus and Host Gene Transcription during Infection.J Virol. 2016 Dec 16;91(1):e01613-16. doi: 10.1128/JVI.01613-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27795423 Free PMC article.
-
ORF7 of Varicella-Zoster Virus Is Required for Viral Cytoplasmic Envelopment in Differentiated Neuronal Cells.J Virol. 2017 May 26;91(12):e00127-17. doi: 10.1128/JVI.00127-17. Print 2017 Jun 15. J Virol. 2017. PMID: 28356523 Free PMC article.
-
[Varicella-zoster virus (VZV)].Uirusu. 2010 Dec;60(2):197-207. doi: 10.2222/jsv.60.197. Uirusu. 2010. PMID: 21488333 Review. Japanese.
-
Molecular and therapeutic aspects of varicella-zoster virus infection.Expert Rev Mol Med. 2005 Aug 10;7(15):1-24. doi: 10.1017/S146239940500966X. Expert Rev Mol Med. 2005. PMID: 16098235 Review.
-
Ability of yeast Ty-VLPs (virus-like particles) containing varicella-zoster virus (VZV)gE and assembly protein fragments to induce in vitro proliferation of human lymphocytes from VZV immune patients.J Med Virol. 1999 Sep;59(1):78-83. J Med Virol. 1999. PMID: 10440812
Cited by
-
Increase in the genetic polymorphism of varicella-zoster virus after passaging in in vitro cell culture.J Microbiol. 2019 Nov;57(11):1033-1039. doi: 10.1007/s12275-019-9429-4. Epub 2019 Oct 28. J Microbiol. 2019. PMID: 31659688
-
The Structures and Functions of VZV Glycoproteins.Curr Top Microbiol Immunol. 2023;438:25-58. doi: 10.1007/82_2021_243. Curr Top Microbiol Immunol. 2023. PMID: 34731265 Free PMC article.
-
Viral modulation of type II interferon increases T cell adhesion and virus spread.Nat Commun. 2024 Jun 22;15(1):5318. doi: 10.1038/s41467-024-49657-4. Nat Commun. 2024. PMID: 38909022 Free PMC article.
-
Chemokine Subversion by Human Herpesviruses.J Innate Immun. 2018;10(5-6):465-478. doi: 10.1159/000492161. Epub 2018 Aug 30. J Innate Immun. 2018. PMID: 30165356 Free PMC article. Review.
-
G-quadruplexes formed by Varicella-Zoster virus reiteration sequences suppress expression of glycoprotein C and regulate viral cell-to-cell spread.PLoS Pathog. 2023 Jan 11;19(1):e1011095. doi: 10.1371/journal.ppat.1011095. eCollection 2023 Jan. PLoS Pathog. 2023. PMID: 36630443 Free PMC article.
References
-
- Roizman B, Pellet PE. Field's Virology. Fifth Edition 2007:2479–99.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

