A first insight into the involvement of phytohormones pathways in coffee resistance and susceptibility to Colletotrichum kahawae
- PMID: 28542545
- PMCID: PMC5438148
- DOI: 10.1371/journal.pone.0178159
A first insight into the involvement of phytohormones pathways in coffee resistance and susceptibility to Colletotrichum kahawae
Abstract
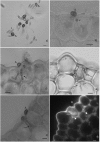
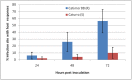
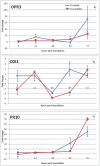
Understanding the molecular mechanisms underlying coffee-pathogen interactions are of key importance to aid disease resistance breeding efforts. In this work the expression of genes involved in salicylic acid (SA), jasmonic acid (JA) and ethylene (ET) pathways were studied in hypocotyls of two coffee varieties challenged with the hemibiotrophic fungus Colletotrichum kahawae, the causal agent of Coffee Berry Disease. Based on a cytological analysis, key time-points of the infection process were selected and qPCR was used to evaluate the expression of phytohormones biosynthesis, reception and responsive-related genes. The resistance to C. kahawae was characterized by restricted fungal growth associated with early accumulation of phenolic compounds in the cell walls and cytoplasmic contents, and deployment of hypersensitive reaction. Similar responses were detected in the susceptible variety, but in a significantly lower percentage of infection sites and with no apparent effect on disease development. Gene expression analysis suggests a more relevant involvement of JA and ET phytohormones than SA in this pathosystem. An earlier and stronger activation of the JA pathway observed in the resistant variety, when compared with the susceptible one, seems to be responsible for the successful activation of defense responses and inhibition of fungal growth. For the ET pathway, the down or non-regulation of ET receptors in the resistant variety, together with a moderate expression of the responsive-related gene ERF1, indicates that this phytohormone may be related with other functions besides the resistance response. However, in the susceptible variety, the stronger activation of ERF1 gene at the beginning of the necrotrophic phase, suggests the involvement of ET in tissue senescence. As far as we know, this is the first attempt to unveil the role of phytohormones in coffee-C. kahawae interactions, thus contributing to deepen our understanding on the complex mechanisms of plant signaling and defense.
Conflict of interest statement
Figures

Similar articles
-
Validation of reference genes for normalization of qPCR gene expression data from Coffea spp. hypocotyls inoculated with Colletotrichum kahawae.BMC Res Notes. 2013 Sep 28;6:388. doi: 10.1186/1756-0500-6-388. BMC Res Notes. 2013. PMID: 24073624 Free PMC article.
-
Epicatechin and catechin may prevent coffee berry disease by inhibition of appressorial melanization of Colletotrichum kahawae.Biotechnol Lett. 2006 Oct;28(20):1637-40. doi: 10.1007/s10529-006-9135-2. Epub 2006 Aug 3. Biotechnol Lett. 2006. PMID: 16955359
-
Transcriptome profiling analysis revealed co-regulation of multiple pathways in jujube during infection by 'Candidatus Phytoplasma ziziphi'.Gene. 2018 Jul 30;665:82-95. doi: 10.1016/j.gene.2018.04.070. Epub 2018 Apr 27. Gene. 2018. PMID: 29709641
-
Mechanisms and strategies of plant defense against Botrytis cinerea.Crit Rev Biotechnol. 2017 Mar;37(2):262-274. doi: 10.1080/07388551.2016.1271767. Epub 2017 Jan 5. Crit Rev Biotechnol. 2017. PMID: 28056558 Review.
-
Directing Trophic Divergence in Plant-Pathogen Interactions: Antagonistic Phytohormones With NO Doubt?Front Plant Sci. 2020 Dec 3;11:600063. doi: 10.3389/fpls.2020.600063. eCollection 2020. Front Plant Sci. 2020. PMID: 33343601 Free PMC article. Review.
Cited by
-
The avocado genome informs deep angiosperm phylogeny, highlights introgressive hybridization, and reveals pathogen-influenced gene space adaptation.Proc Natl Acad Sci U S A. 2019 Aug 20;116(34):17081-17089. doi: 10.1073/pnas.1822129116. Epub 2019 Aug 6. Proc Natl Acad Sci U S A. 2019. PMID: 31387975 Free PMC article.
-
Chickpea Roots Undergoing Colonisation by Phytophthora medicaginis Exhibit Opposing Jasmonic Acid and Salicylic Acid Accumulation and Signalling Profiles to Leaf Hemibiotrophic Models.Microorganisms. 2022 Feb 2;10(2):343. doi: 10.3390/microorganisms10020343. Microorganisms. 2022. PMID: 35208798 Free PMC article.
-
Subcuticular−Intracellular Hemibiotrophy of Colletotrichum lupini in Lupinus mutabilis.Plants (Basel). 2022 Nov 9;11(22):3028. doi: 10.3390/plants11223028. Plants (Basel). 2022. PMID: 36432755 Free PMC article.
-
The Role of Plant Hormones in the Interaction of Colletotrichum Species with Their Host Plants.Int J Mol Sci. 2021 Nov 18;22(22):12454. doi: 10.3390/ijms222212454. Int J Mol Sci. 2021. PMID: 34830343 Free PMC article. Review.
-
Identification of Regulatory Networks of MicroRNAs and Their Targets in Response to Colletotrichum gloeosporioides in Tea Plant (Camellia sinensis L.).Front Plant Sci. 2019 Sep 12;10:1096. doi: 10.3389/fpls.2019.01096. eCollection 2019. Front Plant Sci. 2019. PMID: 31572415 Free PMC article.
References
-
- van der Vossen HAM, Walyaro DJ. Additional evidence for oligogenic inheritance of durable host resistance to coffee berry disease (Colletotrichum kahawae) in arabica coffee (Coffea arabica L.). Euphytica. 2009; 165:105–111.
-
- McDonald L. A preliminary account of a disease of green coffee berries in Kenya Colony. Trans Br Mycol Soc. 1926; 11:145–154.
-
- Silva MC, Várzea V, Guerra-Guimarães L, Azinheira HG, Fernandez D, Petitot A, et al. Coffee resistance to the main diseases : leaf rust and coffee berry disease. Brazilian J Plant Physiol. 2006; 18:119–47.
-
- Firman ID, Waller JM. Coffee berry disease and other Colletotrichum diseases of coffee 4th ed. K Surrey, England: Commonwealth Mycological Institute; 1977.
-
- Hindorf H, Omondi CO. A review of three major fungal diseases of Coffea arabica L. in the rainforests of Ethiopia and progress in breeding for resistance in Kenya. J Adv Res. 2011; 2:109–120.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

