Robustness analysis of the detailed kinetic model of an ErbB signaling network by using dynamic sensitivity
- PMID: 28542548
- PMCID: PMC5443533
- DOI: 10.1371/journal.pone.0178250
Robustness analysis of the detailed kinetic model of an ErbB signaling network by using dynamic sensitivity
Abstract
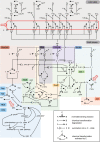
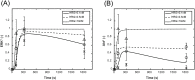
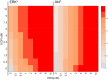
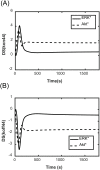
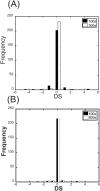
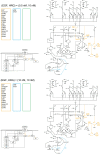
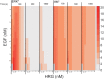
The ErbB receptor signaling pathway plays an important role in the regulation of cellular proliferation, survival and differentiation, and dysregulation of the pathway is linked to various types of human cancer. Mathematical models have been developed as a practical complementary approach to deciphering the complexity of ErbB receptor signaling and elucidating how the pathways discriminate between ligands to induce different cell fates. In this study, we developed a simulator to accurately calculate the dynamic sensitivity of extracellular-signal-regulated kinase (ERK) activity (ERK*) and Akt activity (Akt*), downstream of the ErbB receptors stimulated with epidermal growth factor (EGF) and heregulin (HRG). To demonstrate the feasibility of this simulator, we estimated how the reactions critically responsible for ERK* and Akt* change with time and in response to different doses of EGF and HRG, and predicted that only a small number of reactions determine ERK* and Akt*. ERK* increased steeply with increasing HRG dose until saturation, while showing a gently rising response to EGF. Akt* had a gradual wide-range response to HRG and a blunt response to EGF. Akt* was sensitive to perturbations of intracellular kinetics, while ERK* was more robust due to multiple, negative feedback loops. Overall, the simulator predicted reactions that were critically responsible for ERK* and Akt* in response to the dose of EGF and HRG, illustrated the response characteristics of ERK* and Akt*, and estimated mechanisms for generating robustness in the ErbB signaling network.
Conflict of interest statement
Figures

Similar articles
-
Curcumin attenuates hyperglycemia-driven EGF-induced invasive and migratory abilities of pancreatic cancer via suppression of the ERK and AKT pathways.Oncol Rep. 2019 Jan;41(1):650-658. doi: 10.3892/or.2018.6833. Epub 2018 Oct 30. Oncol Rep. 2019. PMID: 30542713
-
Ligand-dependent responses of the ErbB signaling network: experimental and modeling analyses.Mol Syst Biol. 2007;3:144. doi: 10.1038/msb4100188. Epub 2007 Nov 13. Mol Syst Biol. 2007. PMID: 18004277 Free PMC article.
-
Quantitative transcriptional control of ErbB receptor signaling undergoes graded to biphasic response for cell differentiation.J Biol Chem. 2007 Feb 9;282(6):4045-56. doi: 10.1074/jbc.M608653200. Epub 2006 Dec 1. J Biol Chem. 2007. PMID: 17142811
-
The role of epidermal growth factor and its receptors in mammalian CNS.Cytokine Growth Factor Rev. 2004 Apr-Jun;15(2-3):147-56. doi: 10.1016/j.cytogfr.2004.01.004. Cytokine Growth Factor Rev. 2004. PMID: 15110798 Review.
-
EGF receptor in breast cancer chemoresistance.Adv Exp Med Biol. 2007;608:113-8. doi: 10.1007/978-0-387-74039-3_8. Adv Exp Med Biol. 2007. PMID: 17993236 Review. No abstract available.
References
-
- Thottassery JV, Sun Y, Westbrook L, Rentz SS, Manuvakhova M, et al. (2004) Prolonged extracellular signal-regulated kinase 1/2 activation during fibroblast growth factor 1- or heregulin beta1-induced antiestrogen-resistant growth of breast cancer cells is resistant to mitogen-activated protein/extracellular regulated kinase kinase inhibitors. Cancer Res 64: 4637–4647. 10.1158/0008-5472.CAN-03-2645 - DOI - PubMed
-
- Kholodenko BN, Demin OV, Moehren G, Hoek JB (1999) Quantification of short term signaling by the epidermal growth factor receptor. J Biol Chem 274: 30169–30181. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

