Impaired functional capacity of fetal endothelial cells in preeclampsia
- PMID: 28542561
- PMCID: PMC5441640
- DOI: 10.1371/journal.pone.0178340
Impaired functional capacity of fetal endothelial cells in preeclampsia
Abstract
Objectives: Preeclampsia is one of the main contributers to maternal and fetal morbidity and mortality during pregnancy. A history of preeclampsia puts mother and offspring at an increased cardiovascular risk in later life. We hypothesized that at the time of birth functional impairments of fetal endothelial cells can be detected in pregnancies complicated by preeclampsia and that a therapeutic intervention using 1,25 (OH)2 vitamin D3 can reverse the adverse effects of preeclampsia on cell function.
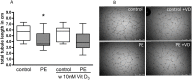
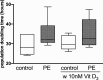
Methods: Human umbilical vein endothelial cells (HUVEC) were isolated from umbilical cords obtained from preeclamptic (N = 12) and uncomplicated pregnancies (N = 13, control). Placental villous tissue fragments from uncomplicated term pregnancies were incubated in explant culture for 48 h at 2% (hypoxia), 8% or 21% O2. Explant conditioned media (CM) was collected and pooled according to oxygen level. We compared the ability of preeclampsia vs. control HUVEC to migrate, proliferate, and form tubule-like networks in a Matrigel assay, in the presence/absence of CM and 1,25(OH)2 vitamin D3.
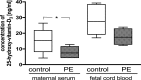
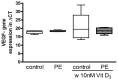
Results: HUVEC from preeclamptic pregnancies showed reduced migration (P = 0.04) and tubule formation (P = 0.04), but no change in proliferation (P = 0.16) compared to healthy pregnancies. Placental villous explant CM derived from 2% O2 incubations significantly reduced HUVEC migration, when compared to non-CM (P = 0.04). Vitamin D3 improved HUVEC function in neither of the groups. There was no significant difference in VEGF gene expression between healthy and preeclamptic pregnancies and no effect of Vitamin D3 on VEGF expression.
Conclusions: Reduced functional abilities of fetal endothelial cells from preeclamptic pregnancies suggests that disease pathways, possibly originating from the dysfunctional placenta, negatively impact fetal endothelium. The neutral effect of 1,25(OH)2 vitamin D3 contrasts with previous findings that vitamin D rescues the poor migration, proliferation and tubule formation exhibited by cord blood fetal endothelial progenitor cells from preeclamptic pregnancies. Further investigations to distinguish pathways by which offspring exposed to preeclampsia are at risk for cardiovascular disease are needed.
Conflict of interest statement
Figures
Similar articles
-
Vitamin D prevents endothelial progenitor cell dysfunction induced by sera from women with preeclampsia or conditioned media from hypoxic placenta.PLoS One. 2014 Jun 2;9(6):e98527. doi: 10.1371/journal.pone.0098527. eCollection 2014. PLoS One. 2014. PMID: 24887145 Free PMC article.
-
Vitamin D antagonizes negative effects of preeclampsia on fetal endothelial colony forming cell number and function.PLoS One. 2014 Jun 3;9(6):e98990. doi: 10.1371/journal.pone.0098990. eCollection 2014. PLoS One. 2014. PMID: 24892558 Free PMC article.
-
Role of vitamin D in cell-cell interaction of fetal endothelial progenitor cells and umbilical cord endothelial cells in a preeclampsia-like model.Am J Physiol Cell Physiol. 2019 Aug 1;317(2):C348-C357. doi: 10.1152/ajpcell.00109.2019. Epub 2019 Jun 5. Am J Physiol Cell Physiol. 2019. PMID: 31166709
-
Placental calcitriol synthesis and IGF-I levels in normal and preeclamptic pregnancies.J Steroid Biochem Mol Biol. 2014 Oct;144 Pt A:44-9. doi: 10.1016/j.jsbmb.2013.12.014. Epub 2013 Dec 24. J Steroid Biochem Mol Biol. 2014. PMID: 24373797 Review.
-
A hypothesis for preeclampsia: adenosine and inducible nitric oxide synthase in human placental microvascular endothelium.Placenta. 2008 Jun;29(6):469-83. doi: 10.1016/j.placenta.2008.02.008. Epub 2008 Mar 24. Placenta. 2008. PMID: 18359514 Review.
Cited by
-
Differential miR-346 and miR-582-3p Expression in Association with Selected Maternal and Fetal Complications.Int J Mol Sci. 2017 Jul 19;18(7):1570. doi: 10.3390/ijms18071570. Int J Mol Sci. 2017. PMID: 28753968 Free PMC article.
-
Sexual Dimorphisms of Preeclampsia-Dysregulated Transcriptomic Profiles and Cell Function in Fetal Endothelial Cells.Hypertension. 2019 Jul;74(1):154-163. doi: 10.1161/HYPERTENSIONAHA.118.12569. Epub 2019 Jun 3. Hypertension. 2019. PMID: 31154903 Free PMC article.
-
Vitamin D-Related Risk Factors for Maternal Morbidity during Pregnancy: A Systematic Review.Nutrients. 2022 Jul 31;14(15):3166. doi: 10.3390/nu14153166. Nutrients. 2022. PMID: 35956342 Free PMC article.
-
Maternal, Fetal, and Placental Selectins in Women With Pre-eclampsia; Association With the Renin-Angiotensin-System.Front Med (Lausanne). 2020 Jun 12;7:270. doi: 10.3389/fmed.2020.00270. eCollection 2020. Front Med (Lausanne). 2020. PMID: 32596247 Free PMC article.
-
GNA11 differentially mediates fibroblast growth factor 2- and vascular endothelial growth factor A-induced cellular responses in human fetoplacental endothelial cells.J Physiol. 2018 Jun;596(12):2333-2344. doi: 10.1113/JP275677. Epub 2018 May 12. J Physiol. 2018. PMID: 29659033 Free PMC article.
References
-
- Davis EF, Lazdam M, Lewandowski AJ, Worton SA, Kelly B, Kenworthy Y, et al. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics. 2012;129(6):e1552–61. doi: 10.1542/peds.2011-3093 - DOI - PubMed
-
- Lazdam M, de la Horra A, Pitcher A, Mannie Z, Diesch J, Trevitt C, et al. Elevated blood pressure in offspring born premature to hypertensive pregnancy: is endothelial dysfunction the underlying vascular mechanism? Hypertension. 2010;56(1):159–65. Epub 2010/05/19. doi: 10.1161/HYPERTENSIONAHA.110.150235 - DOI - PubMed
-
- Jayet PY, Rimoldi SF, Stuber T, Salmon CS, Hutter D, Rexhaj E, et al. Pulmonary and systemic vascular dysfunction in young offspring of mothers with preeclampsia. Circulation. 2010;122(5):488–94. Epub 2010/07/21. doi: 10.1161/CIRCULATIONAHA.110.941203 - DOI - PubMed
-
- Kajantie E, Phillips DI, Andersson S, Barker DJ, Dunkel L, Forsen T, et al. Size at birth, gestational age and cortisol secretion in adult life: foetal programming of both hyper- and hypocortisolism? Clin Endocrinol (Oxf). 2002;57(5):635–41. Epub 2002/10/23. - PubMed
-
- Kajantie E, Eriksson JG, Osmond C, Thornburg K, Barker DJ. Pre-eclampsia is associated with increased risk of stroke in the adult offspring: the Helsinki birth cohort study. Stroke. 2009;40(4):1176–80. Epub 2009/03/07. doi: 10.1161/STROKEAHA.108.538025 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

