Identification and characteristics of extracellular vesicles from bovine blastocysts produced in vitro
- PMID: 28542562
- PMCID: PMC5444795
- DOI: 10.1371/journal.pone.0178306
Identification and characteristics of extracellular vesicles from bovine blastocysts produced in vitro
Abstract
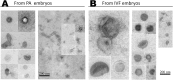
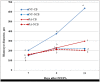
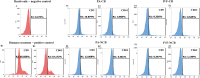
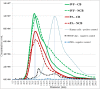
Extracellular vesicles (EVs) have been identified within different body fluids and cell culture media. However, there is very little information on the secretion of these vesicles during early embryonic development. The aims of this work were first to demonstrate the secretion of extracellular vesicles by pre-implantation bovine embryos and second to identify and characterize the population of EVs secreted by bovine blastocysts during the period from day seven to nine of embryo culture and its correlation with further embryo development up to day 11. Bovine embryos were produced by in vitro fertilization (IVF) or parthenogenetic activation (PA) and cultured until blastocyst stage. Blastocyst selection was performed at day 7 post IVF/PA considering two variables: stage of development and quality of embryos. Selected blastocysts were cultured in vitro for 48 hours in groups (exp. 1) or individually (exp. 2) in SOF media depleted of exosomes. At day 9 post IVF/PA the media was collected and EVs isolated by ultracentrifugation. Transmission electron microscopy revealed the presence of heterogeneous vesicles of different sizes and population: microvesicles (MVs) and exosomes (EXs) of rounded shape, enclosed by a lipid bi-layer and ranging from 30 to 385 nm of diameter. Flow cytometry analysis allowed identifying CD63 and CD9 proteins as exosome markers. Nanoparticle tracking analysis generated a large number of variables, which required the use of multivariate statistics. The results indicated that the concentration of vesicles is higher in those blastocysts with arrested development from day 9 up to day 11 of in vitro development (6.7 x 108 particles/ml) derived from IVF (p <0.05), compared to PA blastocysts (4.7 x 108 particles/ml). Likewise, the profile (concentration and diameter) of particles secreted by embryos derived from IVF were different from those secreted by PA embryos. In conclusion, we demonstrated that bovine blastocysts secrete MVs/EXs to the culture media. Data suggest that characteristics of the population of EVs vary depending on embryo competence.
Conflict of interest statement
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

