Combination therapy of cancer with cancer vaccine and immune checkpoint inhibitors: A mathematical model
- PMID: 28542574
- PMCID: PMC5444846
- DOI: 10.1371/journal.pone.0178479
Combination therapy of cancer with cancer vaccine and immune checkpoint inhibitors: A mathematical model
Abstract
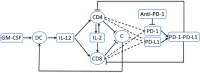
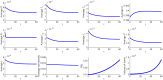
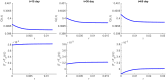
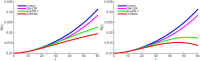
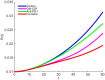
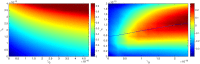
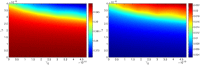
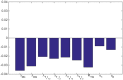
In this paper we consider a combination therapy of cancer. One drug is a vaccine which activates dendritic cells so that they induce more T cells to infiltrate the tumor. The other drug is a checkpoint inhibitor, which enables the T cells to remain active against the cancer cells. The two drugs are positively correlated in the sense that an increase in the amount of each drug results in a reduction in the tumor volume. We consider the question whether a treatment with combination of the two drugs at certain levels is preferable to a treatment by one of the drugs alone at 'roughly' twice the dosage level; if that is the case, then we say that there is a positive 'synergy' for this combination of dosages. To address this question, we develop a mathematical model using a system of partial differential equations. The variables include dendritic and cancer cells, CD4+ and CD8+ T cells, IL-12 and IL-2, GM-CSF produced by the vaccine, and a T cell checkpoint inhibitor associated with PD-1. We use the model to explore the efficacy of the two drugs, separately and in combination, and compare the simulations with data from mouse experiments. We next introduce the concept of synergy between the drugs and develop a synergy map which suggests in what proportion to administer the drugs in order to achieve the maximum reduction of tumor volume under the constraint of maximum tolerated dose.
Conflict of interest statement
Figures
Similar articles
-
Sequential Anti-PD1 Therapy Following Dendritic Cell Vaccination Improves Survival in a HER2 Mammary Carcinoma Model and Identifies a Critical Role for CD4 T Cells in Mediating the Response.Front Immunol. 2019 Aug 14;10:1939. doi: 10.3389/fimmu.2019.01939. eCollection 2019. Front Immunol. 2019. PMID: 31475002 Free PMC article.
-
PD-1 blockade enhances the antitumor efficacy of GM-CSF surface-modified bladder cancer stem cells vaccine.Int J Cancer. 2018 May 15;142(10):2106-2117. doi: 10.1002/ijc.31219. Epub 2017 Dec 26. Int J Cancer. 2018. PMID: 29243219
-
The boosting effect of co-transduction with cytokine genes on cancer vaccine therapy using genetically modified dendritic cells expressing tumor-associated antigen.Int J Oncol. 2006 Apr;28(4):947-53. Int J Oncol. 2006. PMID: 16525645
-
Cellular immunotherapy using irradiated lung cancer cell vaccine co-expressing GM-CSF and IL-18 can induce significant antitumor effects.BMC Cancer. 2014 Jan 29;14:48. doi: 10.1186/1471-2407-14-48. BMC Cancer. 2014. Retraction in: BMC Cancer. 2023 Jul 3;23(1):612. doi: 10.1186/s12885-023-11123-7. PMID: 24475975 Free PMC article. Retracted.
-
GM-CSF-secreting vaccines for solid tumors.Curr Opin Investig Drugs. 2009 Dec;10(12):1315-24. Curr Opin Investig Drugs. 2009. PMID: 19943203 Review.
Cited by
-
Sex-Related Differences in the Immune System Drive Differential Responses to Anti-PD-1 Immunotherapy.Biomolecules. 2024 Nov 27;14(12):1513. doi: 10.3390/biom14121513. Biomolecules. 2024. PMID: 39766221 Free PMC article.
-
Chronic hepatitis B virus and liver fibrosis: A mathematical model.PLoS One. 2018 Apr 10;13(4):e0195037. doi: 10.1371/journal.pone.0195037. eCollection 2018. PLoS One. 2018. PMID: 29634771 Free PMC article.
-
Mathematical modeling of intratumoral immunotherapy yields strategies to improve the treatment outcomes.PLoS Comput Biol. 2023 Dec 19;19(12):e1011740. doi: 10.1371/journal.pcbi.1011740. eCollection 2023 Dec. PLoS Comput Biol. 2023. PMID: 38113269 Free PMC article.
-
A comprehensive review about the utilization of immune checkpoint inhibitors and combination therapy in hepatocellular carcinoma: an updated review.Cancer Cell Int. 2022 Aug 23;22(1):269. doi: 10.1186/s12935-022-02682-z. Cancer Cell Int. 2022. PMID: 35999569 Free PMC article. Review.
-
Mathematical Modelling Based on In Vivo Imaging Suggests CD137-Stimulated Cytotoxic T Lymphocytes Exert Superior Tumour Control Due to an Enhanced Antimitotic Effect on Tumour Cells.Cancers (Basel). 2021 May 24;13(11):2567. doi: 10.3390/cancers13112567. Cancers (Basel). 2021. PMID: 34073822 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

