Highly efficient maternal-fetal Zika virus transmission in pregnant rhesus macaques
- PMID: 28542585
- PMCID: PMC5444831
- DOI: 10.1371/journal.ppat.1006378
Highly efficient maternal-fetal Zika virus transmission in pregnant rhesus macaques
Abstract
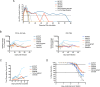
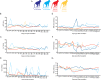
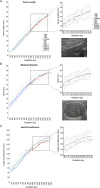
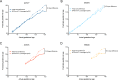
Infection with Zika virus (ZIKV) is associated with human congenital fetal anomalies. To model fetal outcomes in nonhuman primates, we administered Asian-lineage ZIKV subcutaneously to four pregnant rhesus macaques. While non-pregnant animals in a previous study contemporary with the current report clear viremia within 10-12 days, maternal viremia was prolonged in 3 of 4 pregnancies. Fetal head growth velocity in the last month of gestation determined by ultrasound assessment of head circumference was decreased in comparison with biparietal diameter and femur length within each fetus, both within normal range. ZIKV RNA was detected in tissues from all four fetuses at term cesarean section. In all pregnancies, neutrophilic infiltration was present at the maternal-fetal interface (decidua, placenta, fetal membranes), in various fetal tissues, and in fetal retina, choroid, and optic nerve (first trimester infection only). Consistent vertical transmission in this primate model may provide a platform to assess risk factors and test therapeutic interventions for interruption of fetal infection. The results may also suggest that maternal-fetal ZIKV transmission in human pregnancy may be more frequent than currently appreciated.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Comment in
-
Congenital Zika virus infection: More than just microcephaly.Sci Transl Med. 2017 Jun 7;9(393):eaan8195. doi: 10.1126/scitranslmed.aan8195. Sci Transl Med. 2017. PMID: 28592568
Similar articles
-
Infection of the maternal-fetal interface and vertical transmission following low-dose inoculation of pregnant rhesus macaques (Macaca mulatta) with an African-lineage Zika virus.PLoS One. 2023 May 4;18(5):e0284964. doi: 10.1371/journal.pone.0284964. eCollection 2023. PLoS One. 2023. PMID: 37141276 Free PMC article.
-
Zika Virus Infects Early- and Midgestation Human Maternal Decidual Tissues, Inducing Distinct Innate Tissue Responses in the Maternal-Fetal Interface.J Virol. 2017 Jan 31;91(4):e01905-16. doi: 10.1128/JVI.01905-16. Print 2017 Feb 15. J Virol. 2017. PMID: 27974560 Free PMC article.
-
African-Lineage Zika Virus Replication Dynamics and Maternal-Fetal Interface Infection in Pregnant Rhesus Macaques.J Virol. 2021 Jul 26;95(16):e0222020. doi: 10.1128/JVI.02220-20. Epub 2021 Jul 26. J Virol. 2021. PMID: 34076485 Free PMC article.
-
Zika Virus Infection in Pregnancy, Microcephaly, and Maternal and Fetal Health: What We Think, What We Know, and What We Think We Know.Arch Pathol Lab Med. 2017 Jan;141(1):26-32. doi: 10.5858/arpa.2016-0382-RA. Epub 2016 Sep 16. Arch Pathol Lab Med. 2017. PMID: 27636525 Review.
-
Using Macaques to Address Critical Questions in Zika Virus Research.Annu Rev Virol. 2019 Sep 29;6(1):481-500. doi: 10.1146/annurev-virology-092818-015732. Epub 2019 Jun 10. Annu Rev Virol. 2019. PMID: 31180813 Free PMC article. Review.
Cited by
-
Transplacental Antibody Transfer of Respiratory Syncytial Virus Specific IgG in Non-Human Primate Mother-Infant Pairs.Pathogens. 2021 Nov 5;10(11):1441. doi: 10.3390/pathogens10111441. Pathogens. 2021. PMID: 34832599 Free PMC article.
-
Dengue Virus Immunity Increases Zika Virus-Induced Damage during Pregnancy.Immunity. 2019 Mar 19;50(3):751-762.e5. doi: 10.1016/j.immuni.2019.01.005. Epub 2019 Feb 5. Immunity. 2019. PMID: 30737148 Free PMC article.
-
Non-human Primate Models to Investigate Mechanisms of Infection-Associated Fetal and Pediatric Injury, Teratogenesis and Stillbirth.Front Genet. 2021 Jul 5;12:680342. doi: 10.3389/fgene.2021.680342. eCollection 2021. Front Genet. 2021. PMID: 34290739 Free PMC article. Review.
-
Zika Virus Causes Persistent Infection in Porcine Conceptuses and may Impair Health in Offspring.EBioMedicine. 2017 Nov;25:73-86. doi: 10.1016/j.ebiom.2017.09.021. Epub 2017 Sep 21. EBioMedicine. 2017. PMID: 29097124 Free PMC article.
-
Nonhuman Primate Models of Zika Virus Infection, Immunity, and Therapeutic Development.J Infect Dis. 2017 Dec 16;216(suppl_10):S928-S934. doi: 10.1093/infdis/jix540. J Infect Dis. 2017. PMID: 29267926 Free PMC article. Review.
References
-
- Musso D, Gubler DJ. Zika Virus. Clin Microbiol Rev. 2016;29(3):487–524. doi: 10.1128/CMR.00072-15 - DOI - PMC - PubMed
-
- Brooks JT, Friedman A, Kachur RE, LaFlam M, Peters PJ, Jamieson DJ. Update: Interim Guidance for Prevention of Sexual Transmission of Zika Virus—United States, July 2016. MMWR Morb Mortal Wkly Rep. 2016;65(29):745–7. doi: 10.15585/mmwr.mm6529e2 - DOI - PubMed
-
- Davidson A, Slavinski S, Komoto K, Rakeman J, Weiss D. Suspected Female-to-Male Sexual Transmission of Zika Virus—New York City, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(28):716–7. doi: 10.15585/mmwr.mm6528e2 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

