Antigen delivery to dendritic cells shapes human CD4+ and CD8+ T cell memory responses to Staphylococcus aureus
- PMID: 28542586
- PMCID: PMC5444865
- DOI: 10.1371/journal.ppat.1006387
Antigen delivery to dendritic cells shapes human CD4+ and CD8+ T cell memory responses to Staphylococcus aureus
Abstract
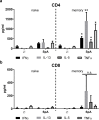
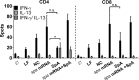
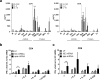
Intracellular persistence of Staphylococcus aureus favors bacterial spread and chronic infections. Here, we provide evidence for the existence of human CD4+ and CD8+ T cell memory against staphylococcal antigens. Notably, the latter could provide a missing link in our understanding of immune control of intracellular S. aureus. The analyses showed that pulsing of monocyte-derived dendritic cells (MoDC) with native staphylococcal protein antigens induced release of Th2-associated cytokines and mediators linked to T regulatory cell development (G-CSF, IL-2 and IL-10) from both CD4+ and CD8+ T cells, thus revealing a state of tolerance predominantly arising from preformed memory T cells. Furthermore, G-CSF was identified as a suppressor of CD8+ T cell-derived IFNγ secretion, thus confirming a tolerogenic role of this cytokine in the regulation of T cell responses to S. aureus. Nevertheless, delivery of in vitro transcribed mRNA-encoded staphylococcal antigens triggered Th1-biased responses, e.g. IFNγ and TNF release from both naïve and memory T cells. Collectively, our data highlight the potential of mRNA-adjuvanted antigen presentation to enable inflammatory responses, thus overriding the existing Th2/Treg-biased memory T cell response to native S. aureus antigens.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Staphylococcus aureus PSM Peptides Modulate Human Monocyte-Derived Dendritic Cells to Prime Regulatory T Cells.Front Immunol. 2018 Nov 13;9:2603. doi: 10.3389/fimmu.2018.02603. eCollection 2018. Front Immunol. 2018. PMID: 30555457 Free PMC article.
-
Nonantigen specific CD8+ T suppressor lymphocytes originate from CD8+CD28- T cells and inhibit both T-cell proliferation and CTL function.Hum Immunol. 2004 Feb;65(2):142-56. doi: 10.1016/j.humimm.2003.12.001. Hum Immunol. 2004. PMID: 14969769
-
CD8α- conventional dendritic cells control Vβ T-cell immunity in response to Staphylococcus aureus infection in mice.Immunology. 2020 Apr;159(4):404-412. doi: 10.1111/imm.13171. Epub 2020 Jan 21. Immunology. 2020. PMID: 31909831 Free PMC article.
-
Interleukin-2 in the development and control of inflammatory disease.Immunol Rev. 2008 Dec;226:19-28. doi: 10.1111/j.1600-065X.2008.00697.x. Immunol Rev. 2008. PMID: 19161413 Review.
-
Immunoglobulin-like transcript 3: A crucial regulator of dendritic cell function.Hum Immunol. 2009 May;70(5):340-4. doi: 10.1016/j.humimm.2009.03.004. Epub 2009 Mar 9. Hum Immunol. 2009. PMID: 19275918 Review.
Cited by
-
Development of a vaccine against Staphylococcus aureus invasive infections: Evidence based on human immunity, genetics and bacterial evasion mechanisms.FEMS Microbiol Rev. 2020 Jan 1;44(1):123-153. doi: 10.1093/femsre/fuz030. FEMS Microbiol Rev. 2020. PMID: 31841134 Free PMC article. Review.
-
Immune Polarization Potential of the S. aureus Virulence Factors SplB and GlpQ and Modulation by Adjuvants.Front Immunol. 2021 Apr 15;12:642802. doi: 10.3389/fimmu.2021.642802. eCollection 2021. Front Immunol. 2021. PMID: 33936060 Free PMC article.
-
The Influence of Antibiotic Resistance on Innate Immune Responses to Staphylococcus aureus Infection.Antibiotics (Basel). 2022 Apr 19;11(5):542. doi: 10.3390/antibiotics11050542. Antibiotics (Basel). 2022. PMID: 35625186 Free PMC article. Review.
-
Inducible Costimulator Contributes to Methicillin-Resistant Staphylococcus aureus Pneumonia.J Infect Dis. 2018 Jul 13;218(4):659-668. doi: 10.1093/infdis/jix664. J Infect Dis. 2018. PMID: 29378030 Free PMC article.
-
Staphylococcus aureus Protein A Induces Human Regulatory T Cells Through Interaction With Antigen-Presenting Cells.Front Immunol. 2020 Sep 30;11:581713. doi: 10.3389/fimmu.2020.581713. eCollection 2020. Front Immunol. 2020. PMID: 33117390 Free PMC article.
References
-
- Eiff C von, Becker K, Machka K, Stammer H, Peters G (2001) Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. The New England journal of medicine 344 (1): 11–16. doi: 10.1056/NEJM200101043440102 - DOI - PubMed
-
- Foster TJ (2005) Immune evasion by staphylococci. Nature reviews. Microbiology 3 (12): 948–958. doi: 10.1038/nrmicro1289 - DOI - PubMed
-
- Botelho-Nevers E, Verhoeven P, Paul S, Grattard F, Pozzetto B et al. (2013) Staphylococcal vaccine development: review of past failures and plea for a future evaluation of vaccine efficacy not only on staphylococcal infections but also on mucosal carriage. Expert review of vaccines 12 (11): 1249–1259. doi: 10.1586/14760584.2013.840091 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

