Seminal plasma induces inflammation and enhances HIV-1 replication in human cervical tissue explants
- PMID: 28542587
- PMCID: PMC5453613
- DOI: 10.1371/journal.ppat.1006402
Seminal plasma induces inflammation and enhances HIV-1 replication in human cervical tissue explants
Erratum in
-
Correction: Seminal plasma induces inflammation and enhances HIV-1 replication in human cervical tissue explants.PLoS Pathog. 2017 Jul 11;13(7):e1006492. doi: 10.1371/journal.ppat.1006492. eCollection 2017 Jul. PLoS Pathog. 2017. PMID: 28700681 Free PMC article.
Abstract
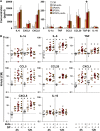
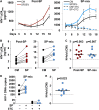
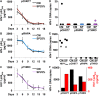
The most immediate and evident effect of mucosal exposure to semen in vivo is a local release of proinflammatory mediators accompanied by an influx of leukocytes into the female genital mucosa (FGM). The implication of such response in HIV-1 transmission has never been addressed due to limitations of currently available experimental models. Using human tissue explants from the uterine cervix, we developed a system of mucosal exposure to seminal plasma (SP) that supports HIV-1 replication. Treatment of ectocervical explants with SP resulted in the upregulation of inflammatory and growth factors, including IL-6, TNF, CCL5, CCL20, CXCL1, and CXCL8, and IL1A, CSF2, IL7, PTGS2, as evaluated by measuring protein levels in explant conditioned medium (ECM) and gene expression in tissue. SP treatment was also associated with increased recruitment of monocytes and neutrophils, as observed upon incubation of peripheral blood leukocytes with ECM in a transwell system. To evaluate the impact of the SP-mediated response on local susceptibility to HIV-1, we infected ectocervical explants with the CCR5-tropic variant HIV-1BaL either in the presence of SP, or after explant pre-incubation with SP. In both experimental settings SP enhanced virus replication as evaluated by HIV-1 p24gag released in explant culture medium over time, as well as by HIV-1 DNA quantification in explants infected in the presence of SP. These results suggest that a sustained inflammatory response elicited by SP soon after coitus may promote HIV-1 transmission to the FGM. Nevertheless, ectocervical tissue explants did not support the replication of transmitted/founder HIV-1 molecular clones, regardless of SP treatment. Our system offers experimental and analytical advantages over traditional models of HIV-1 transmission for the study of SP immunoregulatory effect on the FGM, and may provide a useful platform to ultimately identify new determinants of HIV-1 infection at this site.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Hladik F, McElrath MJ (2008) Setting the stage: host invasion by HIV. Nat Rev Immunol 8: 447–457. doi: 10.1038/nri2302 - DOI - PMC - PubMed
-
- Roan NR, Munch J (2015) Improving preclinical models of HIV microbicide efficacy. Trends Microbiol 23: 445–447. doi: 10.1016/j.tim.2015.05.001 - DOI - PMC - PubMed
-
- Southern PJ (2013) Missing out on the biology of heterosexual HIV-1 transmission. Trends Microbiol 21: 245–252. doi: 10.1016/j.tim.2013.02.002 - DOI - PubMed
-
- Vanpouille C, Arakelyan A, Margolis L (2012) Microbicides: still a long road to success. Trends Microbiol 20: 369–375. doi: 10.1016/j.tim.2012.05.005 - DOI - PMC - PubMed
-
- Doncel GF, Anderson S, Zalenskaya I (2014) Role of semen in modulating the female genital tract microenvironment-implications for HIV transmission. Am J Reprod Immunol 71: 564–574. doi: 10.1111/aji.12231 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

