Antifungal Tc17 cells are durable and stable, persisting as long-lasting vaccine memory without plasticity towards IFNγ cells
- PMID: 28542595
- PMCID: PMC5456400
- DOI: 10.1371/journal.ppat.1006356
Antifungal Tc17 cells are durable and stable, persisting as long-lasting vaccine memory without plasticity towards IFNγ cells
Abstract
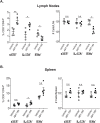
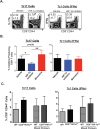
Our understanding of persistence and plasticity of IL-17A+ memory T cells is clouded by conflicting results in models analyzing T helper 17 cells. We studied memory IL-17A+ CD8+ T-cell (Tc17) homeostasis, persistence and plasticity during fungal vaccine immunity. We report that vaccine-induced memory Tc17 cells persist with high fidelity to the type 17 phenotype. Tc17 cells persisted durably for a year as functional IL-17A+ memory cells without converting to IFNγ+ (Tc1) cells, although they produced multiple type I cytokines in the absence of residual vaccine antigen. Memory Tc17 cells were canonical CD8+ T cells with phenotypic features distinct from Tc1 cells, and were Ror(γ)thi, TCF-1hi, T-betlo and EOMESlo. In investigating the bases of Tc17 persistence, we observed that memory Tc17 cells had much higher levels of basal homeostatic proliferation than did Tc1 cells. Conversely, memory Tc17 cells displayed lower levels of anti-apoptotic molecules Bcl-2 and Bcl-xL than Tc1 cells, yet were resistant to apoptosis. Tc1 cells required Bcl-2 for their survival, but Bcl-2 was dispensable for the maintenance of Tc17 cells. Tc17 and Tc1 cells displayed different requirements for HIF-1α during effector differentiation and sustenance and memory persistence. Thus, antifungal vaccination induces durable and stable memory Tc17 cells with distinct requirements for long-term persistence that distinguish them from memory Tc1 cells.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






Similar articles
-
Intrinsic MyD88-Akt1-mTOR Signaling Coordinates Disparate Tc17 and Tc1 Responses during Vaccine Immunity against Fungal Pneumonia.PLoS Pathog. 2015 Sep 14;11(9):e1005161. doi: 10.1371/journal.ppat.1005161. eCollection 2015 Sep. PLoS Pathog. 2015. PMID: 26367276 Free PMC article.
-
Tc17 cells mediate vaccine immunity against lethal fungal pneumonia in immune deficient hosts lacking CD4+ T cells.PLoS Pathog. 2012;8(7):e1002771. doi: 10.1371/journal.ppat.1002771. Epub 2012 Jul 19. PLoS Pathog. 2012. PMID: 22829762 Free PMC article.
-
IL-12 is required for induction but not maintenance of protective, memory responses to Blastomyces dermatitidis: implications for vaccine development in immune-deficient hosts.J Immunol. 2005 Oct 15;175(8):5288-97. doi: 10.4049/jimmunol.175.8.5288. J Immunol. 2005. PMID: 16210634
-
Tc17 Cells in Immunity and Systemic Autoimmunity.Int Rev Immunol. 2015;34(4):318-31. doi: 10.3109/08830185.2014.954698. Epub 2014 Sep 26. Int Rev Immunol. 2015. PMID: 25259411 Review.
-
IL-17A-producing CD8(+)T cells as therapeutic targets in autoimmunity.Expert Opin Ther Targets. 2015 May;19(5):651-61. doi: 10.1517/14728222.2014.997710. Epub 2015 Jan 22. Expert Opin Ther Targets. 2015. PMID: 25611933 Review.
Cited by
-
T cell responses to control fungal infection in an immunological memory lens.Front Immunol. 2022 Sep 13;13:905867. doi: 10.3389/fimmu.2022.905867. eCollection 2022. Front Immunol. 2022. PMID: 36177012 Free PMC article. Review.
-
The Role of the Interleukin-17 Axis and Neutrophils in the Pathogenesis of Endemic and Systemic Mycoses.Front Cell Infect Microbiol. 2020 Dec 14;10:595301. doi: 10.3389/fcimb.2020.595301. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33425780 Free PMC article. Review.
-
PD-1 limits differentiation and plasticity of Tc17 cells.Front Immunol. 2023 Apr 28;14:1104730. doi: 10.3389/fimmu.2023.1104730. eCollection 2023. Front Immunol. 2023. PMID: 37205114 Free PMC article.
-
Fungal immunization potentiates CD4+ T cell-independent cDC2 responses for cross-presentation.Front Immunol. 2025 May 26;16:1602174. doi: 10.3389/fimmu.2025.1602174. eCollection 2025. Front Immunol. 2025. PMID: 40491915 Free PMC article.
-
IL-17A-producing CD8+ T cells promote PDAC via induction of inflammatory cancer-associated fibroblasts.Gut. 2023 Aug;72(8):1510-1522. doi: 10.1136/gutjnl-2022-327855. Epub 2023 Feb 9. Gut. 2023. PMID: 36759154 Free PMC article.
References
-
- Seder RA, Ahmed R (2003) Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol 4: 835–842. doi: 10.1038/ni969 - DOI - PubMed
-
- Miller JD, van der Most RG, Akondy RS, Glidewell JT, Albott S, et al. (2008) Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity 28: 710–722. doi: 10.1016/j.immuni.2008.02.020 - DOI - PubMed
-
- Murali-Krishna K, Lau LL, Sambhara S, Lemonnier F, Altman J, et al. (1999) Persistence of memory CD8 T cells in MHC class I-deficient mice. Science 286: 1377–1381. - PubMed
-
- Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, et al. (2003) Duration of antiviral immunity after smallpox vaccination. Nat Med 9: 1131–1137. doi: 10.1038/nm917 - DOI - PubMed
-
- Surh CD, Sprent J (2008) Homeostasis of naive and memory T cells. Immunity 29: 848–862. doi: 10.1016/j.immuni.2008.11.002 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

