Progressive mitochondrial protein lysine acetylation and heart failure in a model of Friedreich's ataxia cardiomyopathy
- PMID: 28542596
- PMCID: PMC5444842
- DOI: 10.1371/journal.pone.0178354
Progressive mitochondrial protein lysine acetylation and heart failure in a model of Friedreich's ataxia cardiomyopathy
Abstract
Introduction: The childhood heart disease of Friedreich's Ataxia (FRDA) is characterized by hypertrophy and failure. It is caused by loss of frataxin (FXN), a mitochondrial protein involved in energy homeostasis. FRDA model hearts have increased mitochondrial protein acetylation and impaired sirtuin 3 (SIRT3) deacetylase activity. Protein acetylation is an important regulator of cardiac metabolism and loss of SIRT3 increases susceptibility of the heart to stress-induced cardiac hypertrophy and ischemic injury. The underlying pathophysiology of heart failure in FRDA is unclear. The purpose of this study was to examine in detail the physiologic and acetylation changes of the heart that occur over time in a model of FRDA heart failure. We predicted that increased mitochondrial protein acetylation would be associated with a decrease in heart function in a model of FRDA.
Methods: A conditional mouse model of FRDA cardiomyopathy with ablation of FXN (FXN KO) in the heart was compared to healthy controls at postnatal days 30, 45 and 65. We evaluated hearts using echocardiography, cardiac catheterization, histology, protein acetylation and expression.
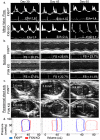
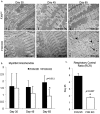
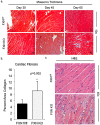
Results: Acetylation was temporally progressive and paralleled evolution of heart failure in the FXN KO model. Increased acetylation preceded detectable abnormalities in cardiac function and progressed rapidly with age in the FXN KO mouse. Acetylation was also associated with cardiac fibrosis, mitochondrial damage, impaired fat metabolism, and diastolic and systolic dysfunction leading to heart failure. There was a strong inverse correlation between level of protein acetylation and heart function.
Conclusion: These results demonstrate a close relationship between mitochondrial protein acetylation, physiologic dysfunction and metabolic disruption in FRDA hypertrophic cardiomyopathy and suggest that abnormal acetylation contributes to the pathophysiology of heart disease in FRDA. Mitochondrial protein acetylation may represent a therapeutic target for early intervention.
Conflict of interest statement
Figures


Similar articles
-
Friedreich's ataxia reveals a mechanism for coordinate regulation of oxidative metabolism via feedback inhibition of the SIRT3 deacetylase.Hum Mol Genet. 2012 Jun 15;21(12):2688-97. doi: 10.1093/hmg/dds095. Epub 2012 Mar 6. Hum Mol Genet. 2012. PMID: 22394676 Free PMC article.
-
Correlation between frataxin expression and contractility revealed by in vitro Friedreich's ataxia cardiac tissue models engineered from human pluripotent stem cells.Stem Cell Res Ther. 2019 Jul 8;10(1):203. doi: 10.1186/s13287-019-1305-y. Stem Cell Res Ther. 2019. PMID: 31286988 Free PMC article.
-
Mitochondrial damage and senescence phenotype of cells derived from a novel frataxin G127V point mutation mouse model of Friedreich's ataxia.Dis Model Mech. 2020 Jul 27;13(7):dmm045229. doi: 10.1242/dmm.045229. Dis Model Mech. 2020. PMID: 32586831 Free PMC article.
-
Biochemistry of cardiomyopathy in the mitochondrial disease Friedreich's ataxia.Biochem J. 2013 Aug 1;453(3):321-36. doi: 10.1042/BJ20130079. Biochem J. 2013. PMID: 23849057 Review.
-
Mitochondrial dysfunction in friedreich's ataxia.Biol Signals Recept. 2001 May-Aug;10(3-4):263-70. doi: 10.1159/000046891. Biol Signals Recept. 2001. PMID: 11351132 Review.
Cited by
-
Acetylation contributes to hypertrophy-caused maturational delay of cardiac energy metabolism.JCI Insight. 2018 May 17;3(10):e99239. doi: 10.1172/jci.insight.99239. eCollection 2018 May 17. JCI Insight. 2018. PMID: 29769443 Free PMC article.
-
Frataxin deficiency induces lipid accumulation and affects thermogenesis in brown adipose tissue.Cell Death Dis. 2020 Jan 23;11(1):51. doi: 10.1038/s41419-020-2253-2. Cell Death Dis. 2020. PMID: 31974344 Free PMC article.
-
Adaptation of the heart to Frataxin depletion: Evidence that integrated stress response can predominate over mTORC1 activation.Hum Mol Genet. 2021 Sep 22;33(8):637-54. doi: 10.1093/hmg/ddab216. Online ahead of print. Hum Mol Genet. 2021. PMID: 34550363 Free PMC article.
-
Harnessing NAD+ Metabolism as Therapy for Cardiometabolic Diseases.Curr Heart Fail Rep. 2022 Aug;19(4):157-169. doi: 10.1007/s11897-022-00550-5. Epub 2022 May 13. Curr Heart Fail Rep. 2022. PMID: 35556214 Free PMC article. Review.
-
Mitochondrial dysfunction in neurodegenerative disorders: Potential therapeutic application of mitochondrial transfer to central nervous system-residing cells.J Transl Med. 2023 Sep 9;21(1):613. doi: 10.1186/s12967-023-04493-w. J Transl Med. 2023. PMID: 37689642 Free PMC article. Review.
References
-
- Mazumder PK, O'Neill BT, Roberts MW, Buchanan J, Yun UJ, Cooksey RC, et al. Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes. 2004;53(9):2366–74. . - PubMed
-
- Turer AT, Stevens RD, Bain JR, Muehlbauer MJ, van der Westhuizen J, Mathew JP, et al. Metabolomic profiling reveals distinct patterns of myocardial substrate use in humans with coronary artery disease or left ventricular dysfunction during surgical ischemia/reperfusion. Circulation. 2009;119(13):1736–46. 10.1161/CIRCULATIONAHA.108.816116 ; - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

