GPR40 partial agonist MK-2305 lower fasting glucose in the Goto Kakizaki rat via suppression of endogenous glucose production
- PMID: 28542610
- PMCID: PMC5441580
- DOI: 10.1371/journal.pone.0176182
GPR40 partial agonist MK-2305 lower fasting glucose in the Goto Kakizaki rat via suppression of endogenous glucose production
Abstract
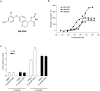
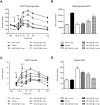
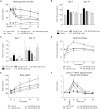
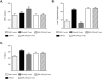
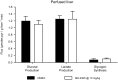
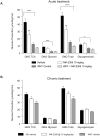
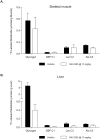
GPR40 (FFA1) is a fatty acid receptor whose activation results in potent glucose lowering and insulinotropic effects in vivo. Several reports illustrate that GPR40 agonists exert glucose lowering in diabetic humans. To assess the mechanisms by which GPR40 partial agonists improve glucose homeostasis, we evaluated the effects of MK-2305, a potent and selective partial GPR40 agonist, in diabetic Goto Kakizaki rats. MK-2305 decreased fasting glucose after acute and chronic treatment. MK-2305-mediated changes in glucose were coupled with increases in plasma insulin during hyperglycemia and glucose challenges but not during fasting, when glucose was normalized. To determine the mechanism(s) mediating these changes in glucose metabolism, we measured the absolute contribution of precursors to glucose production in the presence or absence of MK-2305. MK-2305 treatment resulted in decreased endogenous glucose production (EGP) driven primarily through changes in gluconeogenesis from substrates entering at the TCA cycle. The decrease in EGP was not likely due to a direct effect on the liver, as isolated perfused liver studies showed no effect of MK-2305 ex vivo and GPR40 is not expressed in the liver. Taken together, our results suggest MK-2305 treatment increases glucose stimulated insulin secretion (GSIS), resulting in changes to hepatic substrate handling that improve glucose homeostasis in the diabetic state. Importantly, these data extend our understanding of the underlying mechanisms by which GPR40 partial agonists reduce hyperglycemia.
Conflict of interest statement
Figures
Similar articles
-
Potentiation of insulin secretion and improvement of glucose intolerance by combining a novel G protein-coupled receptor 40 agonist DS-1558 with glucagon-like peptide-1 receptor agonists.Eur J Pharmacol. 2014 Aug 15;737:194-201. doi: 10.1016/j.ejphar.2014.05.014. Epub 2014 May 22. Eur J Pharmacol. 2014. PMID: 24858371
-
GPR40 protein levels are crucial to the regulation of stimulated hormone secretion in pancreatic islets. Lessons from spontaneous obesity-prone and non-obese type 2 diabetes in rats.Mol Cell Endocrinol. 2013 Dec 5;381(1-2):150-9. doi: 10.1016/j.mce.2013.07.025. Epub 2013 Aug 2. Mol Cell Endocrinol. 2013. PMID: 23911664
-
Selective small-molecule agonists of G protein-coupled receptor 40 promote glucose-dependent insulin secretion and reduce blood glucose in mice.Diabetes. 2008 Aug;57(8):2211-9. doi: 10.2337/db08-0130. Epub 2008 May 13. Diabetes. 2008. PMID: 18477808 Free PMC article.
-
GPR40 agonists for the treatment of type 2 diabetes mellitus: The biological characteristics and the chemical space.Bioorg Med Chem Lett. 2016 Dec 1;26(23):5603-5612. doi: 10.1016/j.bmcl.2016.10.074. Epub 2016 Oct 26. Bioorg Med Chem Lett. 2016. PMID: 27825762 Review.
-
Modulating GPR40: therapeutic promise and potential in diabetes.Drug Discov Today. 2013 Dec;18(23-24):1301-8. doi: 10.1016/j.drudis.2013.09.003. Epub 2013 Sep 16. Drug Discov Today. 2013. PMID: 24051395 Review.
Cited by
-
Mukaiyama aldol reaction: an effective asymmetric approach to access chiral natural products and their derivatives/analogues.RSC Adv. 2023 Nov 8;13(47):32975-33027. doi: 10.1039/d3ra05058k. eCollection 2023 Nov 7. RSC Adv. 2023. PMID: 38025859 Free PMC article. Review.
-
Gs/Gq signaling switch in β cells defines incretin effectiveness in diabetes.J Clin Invest. 2020 Dec 1;130(12):6639-6655. doi: 10.1172/JCI140046. J Clin Invest. 2020. PMID: 33196462 Free PMC article.
-
Learn from failures and stay hopeful to GPR40, a GPCR target with robust efficacy, for therapy of metabolic disorders.Front Pharmacol. 2022 Oct 25;13:1043828. doi: 10.3389/fphar.2022.1043828. eCollection 2022. Front Pharmacol. 2022. PMID: 36386134 Free PMC article. Review.
-
Probing Hepatic Glucose Metabolism via 13C NMR Spectroscopy in Perfused Livers-Applications to Drug Development.Metabolites. 2021 Oct 20;11(11):712. doi: 10.3390/metabo11110712. Metabolites. 2021. PMID: 34822370 Free PMC article. Review.
References
-
- Negoro N, Sasaki S, Mikami S, Ito M, Tsujihata Y, Ito R, et al. Optimization of (2,3-dihydro-1-benzofuran-3-yl)acetic acids: discovery of a non-free fatty acid-like, highly bioavailable G protein-coupled receptor 40/free fatty acid receptor 1 agonist as a glucose-dependent insulinotropic agent. J Med Chem. 2012;55: 3960–3974. 10.1021/jm300170m - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

