Platelet proteome reveals novel pathways of platelet activation and platelet-mediated immunoregulation in dengue
- PMID: 28542641
- PMCID: PMC5453622
- DOI: 10.1371/journal.ppat.1006385
Platelet proteome reveals novel pathways of platelet activation and platelet-mediated immunoregulation in dengue
Abstract
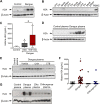
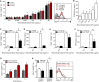
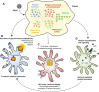
Dengue is the most prevalent human arbovirus disease worldwide. Dengue virus (DENV) infection causes syndromes varying from self-limiting febrile illness to severe dengue. Although dengue pathophysiology is not completely understood, it is widely accepted that increased inflammation plays important roles in dengue pathogenesis. Platelets are blood cells classically known as effectors of hemostasis which have been increasingly recognized to have major immune and inflammatory activities. Nevertheless, the phenotype and effector functions of platelets in dengue pathogenesis are not completely understood. Here we used quantitative proteomics to investigate the protein content of platelets in clinical samples from patients with dengue compared to platelets from healthy donors. Our assays revealed a set of 252 differentially abundant proteins. In silico analyses associated these proteins with key molecular events including platelet activation and inflammatory responses, and with events not previously attributed to platelets during dengue infection including antigen processing and presentation, proteasome activity, and expression of histones. From these results, we conducted functional assays using samples from a larger cohort of patients and demonstrated evidence for platelet activation indicated by P-selectin (CD62P) translocation and secretion of granule-stored chemokines by platelets. In addition, we found evidence that DENV infection triggers HLA class I synthesis and surface expression by a mechanism depending on functional proteasome activity. Furthermore, we demonstrate that cell-free histone H2A released during dengue infection binds to platelets, increasing platelet activation. These findings are consistent with functional importance of HLA class I, proteasome subunits, and histones that we found exclusively in proteome analysis of platelets in samples from dengue patients. Our study provides the first in-depth characterization of the platelet proteome in dengue, and sheds light on new mechanisms of platelet activation and platelet-mediated immune and inflammatory responses.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Similar articles
-
Dengue Virus Induces the Release of sCD40L and Changes in Levels of Membranal CD42b and CD40L Molecules in Human Platelets.Viruses. 2018 Jul 5;10(7):357. doi: 10.3390/v10070357. Viruses. 2018. PMID: 29976871 Free PMC article.
-
Platelet activation and apoptosis modulate monocyte inflammatory responses in dengue.J Immunol. 2014 Aug 15;193(4):1864-72. doi: 10.4049/jimmunol.1400091. Epub 2014 Jul 11. J Immunol. 2014. PMID: 25015827 Free PMC article.
-
Dengue induces platelet activation, mitochondrial dysfunction and cell death through mechanisms that involve DC-SIGN and caspases.J Thromb Haemost. 2013 May;11(5):951-62. doi: 10.1111/jth.12178. J Thromb Haemost. 2013. PMID: 23433144 Free PMC article.
-
Platelets in dengue infection: more than a numbers game.Platelets. 2022 Feb 17;33(2):176-183. doi: 10.1080/09537104.2021.1921722. Epub 2021 May 24. Platelets. 2022. PMID: 34027810 Review.
-
Emerging concepts in dengue pathogenesis: interplay between plasmablasts, platelets, and complement in triggering vasculopathy.Crit Rev Immunol. 2014;34(3):227-40. doi: 10.1615/critrevimmunol.2014010212. Crit Rev Immunol. 2014. PMID: 24941075 Review.
Cited by
-
Potential Pathways and Pathophysiological Implications of Viral Infection-Driven Activation of Kallikrein-Kinin System (KKS).Viruses. 2024 Feb 3;16(2):245. doi: 10.3390/v16020245. Viruses. 2024. PMID: 38400022 Free PMC article. Review.
-
Uncomplicated Plasmodium vivax malaria: mapping the proteome from circulating platelets.Clin Proteomics. 2022 Jan 5;19(1):1. doi: 10.1186/s12014-021-09337-7. Clin Proteomics. 2022. PMID: 34991449 Free PMC article.
-
Platelets: an outlook from biology through evidence-based achievements in critical care.Ann Transl Med. 2017 Nov;5(22):449. doi: 10.21037/atm.2017.11.04. Ann Transl Med. 2017. PMID: 29264366 Free PMC article. Review.
-
Immunoinflammatory markers SIRI and NAR as predictors of respiratory distress syndrome and secondary infections in premature infants.Front Cell Infect Microbiol. 2024 Dec 13;14:1512884. doi: 10.3389/fcimb.2024.1512884. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39735264 Free PMC article.
-
Regulatory T-cells in acute dengue viral infection.Immunology. 2018 May;154(1):89-97. doi: 10.1111/imm.12863. Epub 2017 Dec 18. Immunology. 2018. PMID: 29140541 Free PMC article.
References
-
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, et al. (2013) The global distribution and burden of dengue. Nature 496: 504–507. doi: 10.1038/nature12060 - DOI - PMC - PubMed
-
- Schmidt AC (2010) Response to dengue fever—the good, the bad, and the ugly? N Engl J Med 363: 484–487. doi: 10.1056/NEJMcibr1005904 - DOI - PubMed
-
- WHO (2009) Dengue: guidelines for diagnosis, treatment, prevention and control. - PubMed
-
- Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, et al. (1997) Dengue in the early febrile phase: viremia and antibody responses. J Infect Dis 176: 322–330. - PubMed
-
- Bozza FA, Cruz OG, Zagne SM, Azeredo EL, Nogueira RM, et al. (2008) Multiplex cytokine profile from dengue patients: MIP-1beta and IFN-gamma as predictive factors for severity. BMC Infect Dis 8: 86 doi: 10.1186/1471-2334-8-86 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

