FOLFOXIRI Plus Bevacizumab as Conversion Therapy for Patients With Initially Unresectable Metastatic Colorectal Cancer: A Systematic Review and Pooled Analysis
- PMID: 28542671
- PMCID: PMC5824228
- DOI: 10.1001/jamaoncol.2017.0278
FOLFOXIRI Plus Bevacizumab as Conversion Therapy for Patients With Initially Unresectable Metastatic Colorectal Cancer: A Systematic Review and Pooled Analysis
Abstract
Importance: The combination of fluorouracil, oxaliplatin, and irinotecan plus bevacizumab (FOLFOXIRI-Bev) is an established and effective first-line chemotherapy regimen for metastatic colorectal cancer. However, resection rates of metastases and overall survival with this schedule have never been systematically evaluated in published studies including, but not limited to, the TRIBE (TRIplet plus BEvacizumab) trial.
Objective: To assess the clinical efficacy of FOLFOXIRI-Bev, including outcomes and rates of surgical conversions.
Data sources: A systematic review was conducted in October 2016 in concordance with the PRISMA guidelines of PubMed, the Cochrane Central Register of Controlled Trials, SCOPUS, Web of Science, Google Scholar, CINAHL, Ovid, and EMBASE using the terms FOLFOXIRI and bevacizumab and (colorectal cancer).
Study selection: Clinical trials, retrospective case series, and prospective case series that used FOLFOXIRI-Bev for the treatment of initially unresectable metastatic colorectal cancer in humans were included. Individual case reports and retrospective case series with fewer than 10 patients were excluded.
Data extraction and synthesis: Data were extracted independently by 2 reviewers on a predesigned, standardized form. Ultimately, data were aggregated to obtain the pooled effect size of efficacy, according to the random-effects model and weighted for the number of patients included in each trial.
Main outcomes and measures: Median overall survival and progression-free survival, overall response rates, and rates of R0 surgical conversions and overall surgical conversions.
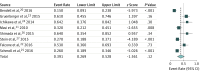
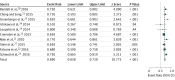
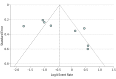
Results: Eleven FOLFOXIRI-Bev studies published between 2010 and 2016 met the inclusion criteria and were pooled for analysis. The studies included 889 patients, with 877 patients clinically evaluable for overall response rates. The objective response rate to FOLFOXIRI-Bev was 69% (95% CI, 65%-72%; I2 = 25%). The rate of overall surgical conversions was 39.1% (95% CI, 26.9%-52.8%), and the rate of R0 surgical conversions was 28.1% (95% CI, 18.1%-40.8%). Median pooled overall survival was 30.2 months (95% CI, 26.5-33.7 months) in 6 trials with data available, and progression-free survival was 12.4 months (95% CI, 10.0-14.3 months) in 9 trials with data available. In meta-regression analysis, variables significantly associated with conversion surgery were disease limited to the liver and a higher median number of cycles (close to 12).
Conclusions and relevance: For patients with surgically unresectable metastatic colorectal cancer, FOLFOXIRI-Bev is associated with a significant overall response rate. Such an effective regimen leads to a probability of surgical conversion of distant metastases approaching 40%, with more than one-fourth of patients having an R0 resection.
Conflict of interest statement
Figures

Similar articles
-
The use of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer: systematic review and economic evaluation.Health Technol Assess. 2008 May;12(15):iii-ix, xi-162. doi: 10.3310/hta12150. Health Technol Assess. 2008. PMID: 18462574
-
Second-line systemic therapy for metastatic colorectal cancer.Cochrane Database Syst Rev. 2017 Jan 27;1(1):CD006875. doi: 10.1002/14651858.CD006875.pub3. Cochrane Database Syst Rev. 2017. PMID: 28128439 Free PMC article.
-
A phase two trial evaluating FOLFIRI plus aflibercept after failure of FOLFOXIRI plus bevacizumab in patients with unresectable metastatic colorectal cancer.Int J Clin Oncol. 2025 Mar;30(3):514-523. doi: 10.1007/s10147-025-02701-9. Epub 2025 Feb 1. Int J Clin Oncol. 2025. PMID: 39891883 Clinical Trial.
-
FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis.Lancet Oncol. 2016 Jun;17(6):801-810. doi: 10.1016/S1470-2045(16)00172-8. Epub 2016 May 6. Lancet Oncol. 2016. PMID: 27160474 Free PMC article.
-
Treatment options for progression or recurrence of glioblastoma: a network meta-analysis.Cochrane Database Syst Rev. 2021 May 4;5(1):CD013579. doi: 10.1002/14651858.CD013579.pub2. Cochrane Database Syst Rev. 2021. PMID: 34559423 Free PMC article.
Cited by
-
Targeting the tumor stroma for cancer therapy.Mol Cancer. 2022 Nov 2;21(1):208. doi: 10.1186/s12943-022-01670-1. Mol Cancer. 2022. PMID: 36324128 Free PMC article. Review.
-
Histopathological and Immune Prognostic Factors in Colo-Rectal Liver Metastases.Cancers (Basel). 2021 Mar 3;13(5):1075. doi: 10.3390/cancers13051075. Cancers (Basel). 2021. PMID: 33802446 Free PMC article. Review.
-
Decylubiquinone Inhibits Colorectal Cancer Growth Through Upregulating Sirtuin2.Front Pharmacol. 2022 Feb 1;12:804265. doi: 10.3389/fphar.2021.804265. eCollection 2021. Front Pharmacol. 2022. PMID: 35177983 Free PMC article.
-
Pathological complete response to mFOLFOX6 plus cetuximab therapy for unresectable colon cancer with multiple paraaortic lymph node metastases.Mol Clin Oncol. 2018 Dec;9(6):587-591. doi: 10.3892/mco.2018.1742. Epub 2018 Oct 5. Mol Clin Oncol. 2018. PMID: 30546885 Free PMC article.
-
Conversion surgery after lenvatinib treatment for multiple lung metastases from hepatocellular carcinoma.Int Cancer Conf J. 2022 Aug 17;12(1):7-13. doi: 10.1007/s13691-022-00567-6. eCollection 2023 Jan. Int Cancer Conf J. 2022. PMID: 36605836 Free PMC article.
References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90. - PubMed
-
- Smith JJ, D’Angelica MI. Surgical management of hepatic metastases of colorectal cancer. Hematol Oncol Clin North Am. 2015;29(1):61-84. - PubMed
-
- Maeda Y, Shinohara T, Nagatsu A, Futakawa N, Hamada T. Long-term outcomes of conversion hepatectomy for initially unresectable colorectal liver metastases. Ann Surg Oncol. 2016;23(suppl 2):S242-S248. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

