Histologic scoring indices for evaluation of disease activity in ulcerative colitis
- PMID: 28542712
- PMCID: PMC6481362
- DOI: 10.1002/14651858.CD011256.pub2
Histologic scoring indices for evaluation of disease activity in ulcerative colitis
Abstract
Background: Disease activity can be determined using clinical, endoscopic or histologic criteria in patients with ulcerative colitis (UC). Persistent disease activity is associated with poor outcomes. Histologic disease activity has been shown to be associated with relapse, colectomy and colorectal cancer. The ability to objectively evaluate microscopic disease activity using histology is important for both clinical practice and clinical trials. However, the operating properties of the currently available histologic indices remain unclear.
Objectives: A systematic review was undertaken to identify and evaluate the development and operating characteristics of histologic disease activity indices used to assess disease activity in people with ulcerative colitis.
Search methods: We searched MEDLINE, EMBASE, PubMed, CENTRAL and the Cochrane IBD Review Group Specialized Trials Register from inception to 2 December 2016 for applicable studies. There were no language or document type restrictions.
Selection criteria: Any study design (e.g. randomized controlled trials, cohort studies, case series) that evaluated a histologic index in patients with UC were considered for inclusion. Eligible patients were adults (> 18 years), diagnosed with UC using conventional clinical, radiographic, endoscopic and histologic criteria.
Data collection and analysis: Two authors (MHM and CEP) independently reviewed the titles and abstracts of the studies identified from the literature search. A standardized form was used to assess eligibility of trials for inclusion and for data extraction.Two authors (MHM and CEP) independently extracted and recorded data, which included the number of patients enrolled, number of patients per treatment arm, patient characteristics including age and gender distribution, and the name of the histologic index. Outcomes (i.e. intra-rater reliability, inter-rater reliability, internal consistency, content validity, criterion validity, construct validity, responsiveness, and feasibility) were recorded for each trial.
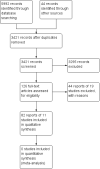
Main results: In total, 126 reports describing 30 scoring indices were identified through the screening process. Eleven of the 30 scoring indices have undergone some form of index validation. Intra-rater reliability was assessed for eight scoring indices. Inter-rater reliability was evaluated for all 11 of the scoring indices. Three of the indices underwent content validation. Two of the included scoring indices assessed criterion validity. Six of the included scoring indices explored content validity. Two of the included scoring indices were tested for responsiveness.
Authors' conclusions: The Nancy Index and the Robarts Histopathology Index have undergone the most validation in that four operating properties including reliability, content validity, construct validity (hypothesis testing) and criterion validity have been tested. However, none of the currently available histologic scoring indices have been fully validated. In order to determine the optimal endpoint for histologic healing in UC, more research is required. The optimal index would need to be fully validated.
Conflict of interest statement
Several authors (MHM, GYZ, BGF, RK, and BGL) were involved in the development of the Robarts score. Robarts Clinical Trials began in 1986 as an academic research unit within the Robarts Research Institute which is affiliated with University Hospital and the University of Western Ontario. All profits from Robarts Clinical Trials, Inc. are directed towards academic research. The University of Western Ontario is the sole shareholder of Robarts Clinical Trials Inc. None of the authors with affiliation to Robarts Clinical Trials, Inc. have an equity position or any shares in the corporation.
Mahmoud H Mosli: None known.
Claire E Parker: None known.
Sigrid A Nelson: None known
Kenneth A Baker: None known.
John K MacDonald: None known.
GY Zou: None known.
Brian G Feagan: Dr Feagan has received fees from Abbott/AbbVie, Amgen, Astra Zeneca, Avaxia Biologics Inc., Bristol‐Myers Squibb, Celgene, Centocor Inc., Elan/Biogen, Ferring, JnJ/Janssen, Merck, Novartis, Novonordisk, Pfizer, Prometheus Laboratories, Protagonist, Salix Pharma, Takeda, Teva, Tillotts Pharma AG, UCB Pharma for Board membership; fees fromAbbott/AbbVie, Actogenix, Albireo Pharma, Amgen, Astra Zeneca, Avaxia Biologics Inc., Axcan, Baxter Healthcare Corp., Boehringer‐Ingelheim, Bristol‐Myers Squibb, Calypso Biotech, Celgene, Elan/Biogen, EnGene, Ferring Pharma, Roche/Genentech, GiCare Pharma, Gilead, Given Imaging Inc., GSK, Ironwood Pharma, Janssen Biotech (Centocor), JnJ/Janssen, Kyowa Kakko Kirin Co Ltd., Lexicon, Lilly, Merck, Millennium, Nektar, Novonordisk, Pfizer, Prometheus Therapeutics and Diagnostics, Protagonist, Receptos, Salix Pharma, Serono, Shire, Sigmoid Pharma, Synergy Pharma Inc., Takeda, Teva Pharma, Tillotts, UCB Pharma, Vertex Pharma, Warner‐Chilcott, Wyeth, Zealand, and Zyngenia for consultancy; and Payment for lectures from Abbott/AbbVie, JnJ/Janssen, Takeda, Warner‐Chilcott, UCB Pharma. All of these financial activities are outside the submitted work.
Reena Khanna: Dr. Khanna has received consulting fees from AbbVie, Janssen, Pfizer, Shire, Takeda. All of these financial activities are outside the submitted work.
Barrett G Levesque: Dr Levesque has received fees from Prometheus Labs, Takeda, Abbvie, Nestle Health Sciences, Gilead, and Roche for consultancy; and payment for lectures from Warner Chilcott, Salix, and UCB Pharma. All of these financial activities are outside the submitted work.
Vipul Jairath: Dr. Jairath has received scientific advisory board fees from Abbvie, Sandoz, Ferring, Janssen, Takeda; speakers fees from Takeda, Janssen, Shire and Ferring; travel support for conference attendance from Vifor pharmaceuticals. All of these financial activities are outside the submitted work.
Figures
Update of
- doi: 10.1002/14651858.CD011256
Similar articles
-
Endoscopic scoring indices for evaluation of disease activity in ulcerative colitis.Cochrane Database Syst Rev. 2018 Jan 16;1(1):CD011450. doi: 10.1002/14651858.CD011450.pub2. Cochrane Database Syst Rev. 2018. PMID: 29338066 Free PMC article.
-
Histologic scoring indices for evaluation of disease activity in Crohn's disease.Cochrane Database Syst Rev. 2017 Jul 21;7(7):CD012351. doi: 10.1002/14651858.CD012351.pub2. Cochrane Database Syst Rev. 2017. PMID: 28731502 Free PMC article.
-
Endoscopic scoring indices for evaluation of disease activity in Crohn's disease.Cochrane Database Syst Rev. 2016 Aug 8;2016(8):CD010642. doi: 10.1002/14651858.CD010642.pub2. Cochrane Database Syst Rev. 2016. PMID: 27501379 Free PMC article.
-
Unfractionated or low-molecular weight heparin for induction of remission in ulcerative colitis.Cochrane Database Syst Rev. 2008 Apr 16;(2):CD006774. doi: 10.1002/14651858.CD006774.pub2. Cochrane Database Syst Rev. 2008. Update in: Cochrane Database Syst Rev. 2010 Oct 06;(10):CD006774. doi: 10.1002/14651858.CD006774.pub3. PMID: 18425969 Updated.
-
Placebo response and remission rates in randomised trials of induction and maintenance therapy for ulcerative colitis.Cochrane Database Syst Rev. 2017 Sep 8;9(9):CD011572. doi: 10.1002/14651858.CD011572.pub2. Cochrane Database Syst Rev. 2017. PMID: 28886205 Free PMC article.
Cited by
-
The impact of clinical symptoms and endoscopic and histologic disease activity on health-related quality of life in patients with ulcerative colitis following treatment with multimatrix mesalazine.Qual Life Res. 2021 Jul;30(7):1925-1938. doi: 10.1007/s11136-021-02787-4. Epub 2021 Mar 2. Qual Life Res. 2021. PMID: 33651279 Free PMC article.
-
A review on the current status and definitions of activity indices in inflammatory bowel disease: how to use indices for precise evaluation.J Gastroenterol. 2022 Apr;57(4):246-266. doi: 10.1007/s00535-022-01862-y. Epub 2022 Mar 2. J Gastroenterol. 2022. PMID: 35235037 Free PMC article. Review.
-
Randomised clinical trial: a phase 1b study of GB004, an oral HIF-1α stabiliser, for treatment of ulcerative colitis.Aliment Pharmacol Ther. 2022 Feb;55(4):401-411. doi: 10.1111/apt.16753. Epub 2022 Jan 10. Aliment Pharmacol Ther. 2022. PMID: 35014040 Free PMC article. Clinical Trial.
-
Correlation of mucosal healing endpoints with long-term clinical and patient-reported outcomes in ulcerative colitis.J Gastroenterol. 2023 Oct;58(10):990-1002. doi: 10.1007/s00535-023-02013-7. Epub 2023 Jul 25. J Gastroenterol. 2023. PMID: 37490069 Free PMC article. Clinical Trial.
-
Role of endoscopic ultrasound as a predictor of histological healing in ulcerative colitis.Ann Med. 2025 Dec;57(1):2499961. doi: 10.1080/07853890.2025.2499961. Epub 2025 Apr 30. Ann Med. 2025. PMID: 40305512 Free PMC article.
References
References to studies included in this review
Feagan 2005 {published data only}
-
- Feagan BG, Greenberg GR, Wild G, Fedorak RN, Pare P, McDonald JW, et al. Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. New England Journal of Medicine 2005;352(24):2499‐507. - PubMed
Fiel 2003 {published data only}
-
- Bouguen G, Levesque BG, Pola S, Evans E, Sandborn WJ. Feasibility of endoscopic assessment and treating to target to achieve mucosal healing in ulcerative colitis. Inflammatory Bowel Diseases 2014;20(2):231‐9. - PubMed
-
- Fiel M, Qin L, Suriawinita A, Xu R, Qui L, Bitar M, et al. Histologic grading of disease activity in chronic IBD: inter‐ and intra‐observer variation among pathologists with different levels of experience. Modern Pathology 2003;83(1):118A.
-
- Gasia MF, Gui X, Panaccione R, Kaplan G, Ghosh S, Lacucci M. Clinical outcome of ulcerative colitis patients with complete mucosal healing but with subtle abnormalities detected by novel iSCAN endoscopic. Gastroenterology 2015;148(4):S826.
-
- Gasia MF, Panaccione R, Kaplan G, Ghosh S, Gui X, Lacucci M. Clinical outcome of ulcerative colitis with mucosal healing demonstrated by white light endoscopy but with subtle abnormalities detected by high definition iSCAN endoscopy. Journal of Crohn's and Colitis 2015;9:S164.
Geboes 2000 {published data only}
-
- Botina A, Shchukina O, Kondrashina E, Kharitidis A, Markova E. Histological examination of colonic mucosa from patients with clinical remission of ulcerative colitis. Virchows Archiv : an International Journal of Pathology 2015;467:S192.
-
- Dargavel C, Kevans D, Kabakchiev B, Riddell RH, Kirsch R, Silverberg MS. Predictive value of basal plasmacytosis on clinical relapse in quiescent ulcerative colitis. Gastroenterology 2015;148(4):S115.
-
- David R, Hertogh G, Jerrold T, Hong W, Ben A, Geert D. Histologic activity after induction therapy with multimatrix mesalamine is associated with 12‐month remission status in ulcerative colitis. Inflammatory Bowel Diseases 2014;20:S69.
-
- Nardo G, Oliva S, Aloi M, Conte F, Viola F, Nuti F, et al. Effectiveness of a rectal infusion of Lactobacillus Reuteri ATCC 55730 in children with distal active ulcerative colitis. Digestive and Liver Disease 2009;41:S233.
-
- Farkas K, Reisz Z, Sejben A, Tiszlavicz L, Szucs M, Szepes, et al. Correlation of histological activity and basal plasmacytosis with mucosal healing in ulcerative colitis patients. Journal of Crohn's and Colitis 2015;9:S183.
Gomes 1986 {published data only}
-
- Bennink R, Peeters M, D'Haens G, Rutgeerts P, Mortelmans L. Tc‐99m HMPAO white blood cell scintigraphy in the assessment of the extent and severity of an acute exacerbation of ulcerative colitis. Clinical Nuclear Medicine 2001;26(2):99‐104. - PubMed
-
- Bennink RJ, Peeters M, Rutgeerts P, Mortelmans L. Evaluation of early treatment response and predicting the need for colectomy in active ulcerative colitis with 99mTc‐HMPAO white blood cell scintigraphy. Journal of Nuclear Medicine 2004;45(10):1698‐704. - PubMed
Jauregui‐Amezaga 2016 {published data only}
-
- Jauregui‐Amezaga A, Das Y, Lobatón T, Lemmens B, Bessissow T, Ferrante M, et al. Validation of the Simplified Geboes Score for Ulcerative Colitis. Journal of Crohn's and Colitis 2016;10:S144. - PubMed
Marchal‐Bressenot 2017 {published data only}
-
- Marchal‐Bressenot A, Salleron J, Boulagnon‐Rombi C, Bastien C, Cahn V, Cadiot G, et al. Development and validation of the Nancy histological index for UC. Gut 2017;66(1):43‐9. - PubMed
Mosli 2017 {published data only}
-
- Mosli MH, Feagan BG, Zou G, Geboes K, Langner C, Peterson MR, et al. Agreement of central readers in the evaluation of histopathological disease activity in ulcerative colitis. Gastroenterology 2014;5(1):S‐225.
-
- Mosli MH, Feagan BG, Zou G, Sandborn WJ, D'Haens G, Khanna R, et al. Development and validation of a histological index for UC. Gut 2017;66(1):50‐8. - PubMed
-
- Mosli MH, Feagan BG, Zou G, Sandborn WJ, D'Haens G, Khanna R, et al. Reproducibility of histological assessments of disease activity in UC. Gut 2015;64(11):1765‐73. - PubMed
Riley 1991 {published data only}
-
- Ando T, Watanabe O, Furuta R, Maeda O, Nishio Y, Nishiwaki T, et al. Predictors of a response to cyclosporine or leukocyte removal therapy in patients with refractory ulcerative colitis. Digestive Endoscopy 2005;17(2):153‐8.
-
- Fukunaga K, Ohda Y, Hida N, Iimuro M, Yokoyama Y, Kamikozuru K, et al. Placebo controlled evaluation of Xilei San, a herbal preparation in patients with intractable ulcerative proctitis. Journal of Gastroenterology and Hepatology 2012;27(12):1808‐15. - PubMed
-
- Langhorst J, Rueffer A, Dobos GJ, Boone JH. Monitoring of histologically defined healing in ulcerative colitis: performance of fecal lactoferrin, calprotectin, PMN‐elastasis and CRP and WBC compared to the Riley score. Gastroenterology 2015;148(4):S448.
-
- Lemmens B, Arijs I, Assche G, Sagaert X, Geboes K, Ferrante M, et al. Correlation between the endoscopic and histologic score in assessing the activity of ulcerative colitis. Inflammatory Bowel Diseases 2013;19(6):1194‐201. - PubMed
Rubin 2007 {published data only}
-
- Rubin D, Keyashian K, Bunnag A, Dave A, Williams J, Hanauer S, et al. Correlation between clinical, endoscopic, and histologic disease activity in ulcerative colitis. American Journal of Gastroenterology 2012;107:S694.
-
- Rubin DT, Cohen RD, Sandborn WJ, Lichtenstein GR, Axler J, Riddell R, et al. Budesonide MMX 9 mg for inducing remission in patients with mild‐to‐moderate ulcerative colitis not adequately controlled with oral 5‐ASAs. Journal of Crohn's & Colitis 2015;9(Suppl 1):S7.
-
- Rubin DT, Huo D, Hetzel JT, Bunnag AP, Sedrak M, Hart JA, et al. Increased degree of histological inflammation predicts colectomy and hospitalisation in patients with ulcerative colitis. Gastroenterology 2007;132(4):A19.
Theede 2015 {published data only}
-
- Theede K, Holck S, Ibsen P, Ladelund S, Nordgaard‐Lassen I, Nielsen AM. Level of fecal calprotectin correlates With endoscopic and histologic inflammation and identifies patients with mucosal healing in ulcerative colitis. Clinical Gastroenterology and Hepatology 2015;13(11):1929‐36. - PubMed
Truelove 1956 {published data only}
-
- Ardizzone S, Petrillo M, Antonacci CM, Bianchi Porro G. Sucralfate and hydrocortisone enemas in the treatment of active ulcerative proctitis‐‐a randomized single‐blind comparative study. Alimentary Pharmacology and Therapeutics 1996;10(6):957‐60. - PubMed
-
- Ardizzone S, Petrillo M, Imbesi V, Cerutti R, Bollani S, Bianchi Porro G. Is maintenance therapy always necessary for patients with ulcerative colitis in remission?. Alimentary Pharmacology and Therapeutics 1999;13(3):373‐9. - PubMed
-
- Bayles T, Sninsky C. Budesonide enema is an effective alternative to hydrocortisone enema in active distal ulcerative colitis. Gastroenterology 1995;108(4 Suppl 2):S778.
-
- Campieri M, Cottone M, Miglio F, Manenti F, Astegiano M, D'Arienzo A, et al. Beclomethasone dipropionate enemas versus prednisolone sodium phosphate enemas in the treatment of distal ulcerative colitis. Alimentary Pharmacology and Therapeutics 1998;12(4):361‐6. - PubMed
References to studies excluded from this review
Baars 2012 {published data only}
-
- Baars JE, Nuij JAA, Oldenburg B, Kuipers EJ, Woude CJ. Majority of patients with inflammatory bowel disease in clinical remission have mucosal inflammation. Inflammatory Bowel Diseases 2012;18(9):1634‐40. - PubMed
D'Argenio 2001 {published data only}
-
- D'Argenio G, Cosenza V, Riegler G, Della Valle N, Deritis F, Mazzacca G. Serum transglutaminase correlates with endoscopic and histopathologic grading in patients with ulcerative colitis. Digestive Diseases and Sciences 2001;46(3):649‐57. - PubMed
-
- Girlich C, Schacherer D, Jung EM, Klebl F, Huber E. Comparison between quantitative assessment of bowel wall vascularization by contrast‐enhanced ultrasound and results of histopathological scoring in ulcerative colitis. International Journal of Colorectal Disease 2012;27(2):193‐8. - PubMed
-
- Scheppach W, Sommer H, Kirchner T, Paganelli GM, Bartram P, Christl S, et al. Effect of butyrate enemas on the colonic mucosa in distal ulcerative colitis. Gastroenterology 1992;103(1):51‐6. - PubMed
Floren 1987 {published data only}
-
- Floren CH, Benoni C, Willen R. Histologic and colonoscopic assessment of disease extension in ulcerative colitis. Scandinavian Journal of Gastroenterology 1987;22(4):459‐62. - PubMed
Friedman 1985 {published data only}
-
- Friedman L, Richter J, Kirkham S. 5‐aminosalicylic acid (5‐ASA) enemasin refractory distal ulcerative colitis (UC): a randomized, controlled trial. Gastroenterology 1985;88:A1388. - PubMed
Gramlich 2007 {published data only}
Hanauer 1993 {published data only}
-
- Hanauer S, Schwartz J, Robinson M, Roufail W, Arora S, Cello J, et al. Mesalamine capsules for treatment of active ulcerative colitis: results of a controlled trial. Pentasa Study Group. American Journal of Gastroenterology 1993;88(8):1188‐97. - PubMed
Iacucci 2015 {published data only}
-
- Gui X, Fort Gasia M, Ghosh S, Iacucci, M. A refined IBD histopathologic grading system (ECAP scheme)‐more sensitive and informative in assessment of mucosal healing. Laboratory Investigation 2014;94:176‐A.
-
- Iacucci M, Fort Gasia M, Hassan C, Panaccione R, Kaplan GG, Ghosh S, et al. Complete mucosal healing defined by endoscopic Mayo subscore still demonstrates abnormalities by novel high definition colonoscopy and refined histological gradings. Endoscopy 2015;47(8):726‐34. - PubMed
Keren 1984 {published data only}
-
- Keren DF, Appelman HD, Dobbins WO III, Wells JJ, Whisenant B, Foley J, et al. Correlation of histopathologic evidence of disease activity with the presence of immunoglobulin containing cells in the colons of patients with inflammatory bowel disease. Human Pathology 1984;15(8):757–63. - PubMed
Korelitz 1976 {published data only}
-
- Korelitz BI, Sommers SC. Responses to drug therapy in ulcerative colitis. American Journal of Digestive Diseases 1976;21(6):441‐7. - PubMed
Matts 1961 {published data only}
-
- Kato K, Mizuno S, Umesaki Y, Ishii Y, Sugitani M, Imaoka A, et al. Randomized placebo‐controlled trial assessing the effect of bifidobacteria‐fermented milk on active ulcerative colitis. Alimentary Pharmacology & Therapeutics 2004;20(10):1133‐41. - PubMed
-
- Kimura K, Kanai T, Bessho R, Hosoe N, Kobayashi T, Takayama T. In vivo visualization and evaluation of colorectal inflammation in ulcerative colitis by a newly integrated endocytoscopy. Gastroenterology 2011;1:S422.
-
- Matts SG. The value of rectal biopsy in the diagnosis of ulcerative colitis. Quarterly Journal of Medicine 1961;30(4):393‐407. - PubMed
-
- Moriichi K, Fujiya M, Utsumi T, Sakatani A, Tanaka K, Dokoshi T. Quantification of autofluorescence imaging is useful for objectively assessing the severity of ulcerative colitis. Gastrointestinal Endoscopy 2015;81(5):AB384‐5.
Nishiyama 2014 {published data only}
-
- Nishiyama S, Tanaka S, Oka S, Shigita K, Asayama N, Sagami, S. Correlation between endocytoscopy and histopathological activity in the remission stage of ulcerative colitis: A pilot study. Gastrointestinal Endoscopy 2014;79(5):AB570‐1.
Odze 1993 {published data only}
-
- Odze R, Antonioli D, Peppercorn M, Goldman H. Effect of topical 5‐aminosalicylic acid (5‐ASA) therapy on rectal mucosal biopsy morphology in chronic ulcerative colitis. American Journal of Surgical Pathology 1993;17(9):869‐75. - PubMed
Powell‐Tuck 1982 {published data only}
-
- Powell‐Tuck J, Day DW, Buckell NA, Wadsworth J, Lennard‐Jones JE. Correlations between defined sigmoidoscopic appearances and other measures of disease activity in ulcerative colitis. Digestive Diseases and Sciences 1982;27(6):533‐7. - PubMed
Riley 1988 {published data only}
-
- Riley SA, Mani V, Goodman MJ, Herd ME, Dutt S, Turnberg LA. Comparison of delayed‐release 5‐aminosalicylic acid (mesalazine) and sulfasalazine as maintenancetreatment for patients with ulcerative colitis. Gastroenterology 1988;94(6):1383‐9. - PubMed
Rutter 2004 {published data only}
-
- Rutter M, Saunders B, Wilkinson K, Rumbles S, Schofield G, Kamm M, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology 2004;126(2):451‐9. - PubMed
Sandborn 1993 {published data only}
-
- Pagnini C, Menasci F, Festa S, Rizzatti G, Corleto VD, Delle Fave M, et al. Application of clinical indexes in ulcerative colitis patients in regular follow‐up visit: correlation with endoscopic 'mucosal healing' and implication for management. Preliminary results. European Review for Medical and Pharmacological Sciences 2015;19(19):3674‐81. - PubMed
-
- Sandborn WJ, Tremaine WJ, Offord KP, Lawson GM, Petersen BT, Batts KP, et al. Transdermal nicotine for mildly to moderately active ulcerative colitis. A randomized, double‐blind, placebo‐controlled trial. Annals of Internal Medicine 1997;126(5):364‐71. - PubMed
-
- Sandborn WJ, Tremaine WJ, Schroeder KW, Steiner BL, Batts KP, Lawson GM. Cyclosporine enemas for treatment‐resistant, mildly to moderately active, left‐sided ulcerative colitis. American Journal of Gastroenterology 1993;88(5):640‐5. - PubMed
Saverymuttu 1986 {published data only}
-
- Berni Canani R, Terrin G, Rapacciuolo L, Miele E, Siani MC, Puzone C, et al. Faecal calprotectin as reliable non‐invasive marker to assess the severity of mucosal inflammation in children with inflammatory bowel disease. Digestive and Liver Disease 2008;40(7):547‐53. - PubMed
-
- Bunn SK, Bisset WM, Main MJ, Gray ES, Olson S, Golden BE. Fecal calprotectin: validation as a noninvasive measure of bowel inflammation in childhood inflammatory bowel disease. Journal of Pediatric Gastroenterology and Nutrition 2001;33(1):14‐22. - PubMed
-
- Celasco G, Papa A, Jones R, Moro L, Bozzella R, Surace M, et al. Clinical trial: oral colon‐release parnaparin sodium tablets (CB‐01‐05 MMX) for active left‐sided ulcerative colitis. Alimentary pharmacology & therapeutics 2010;31(3):375‐86. - PubMed
-
- D'Haens GR, Kovacs A, Vergauwe P, Nagy F, Molnar T, Bouhnik Y, et al. Clinical trial: Preliminary efficacy and safety study of a new Budesonide‐MMX(R) 9 mg extended‐release tablets in patients with active left‐sided ulcerative colitis. Journal of Crohn's and Colitis 2010;4(2):153‐60. - PubMed
-
- Dotan I, Hallak A, Arber N, Santo M, Alexandrowitz A, Knaani Y, et al. Low‐dose low‐molecular weight heparin (enoxaparin) is effective as adjuvant treatment in active ulcerative colitis an open trial. Digestive Diseases and Sciences 2001;46(10):2239‐44. - PubMed
Watts 1966 {published data only}
-
- Almer S, Franzen L, Peters AM, Tjadermo M, Ekberg S, Granerus G, et al. Do technetium‐99m hexamethylpropylene amine oxime‐labeled leukocytes truly reflect the mucosal inflammation in patients with ulcerative colitis?. Scandinavian Journal of Gastroenterology 1992;27(12):1031‐8. - PubMed
Wiernicka 2015 {published data only}
-
- Szymanska S, Wiernicka A, Cielecka‐Kuszyk J, Szymanska E, Dadalski M, Kierkus, J. Influence of induction therapy with infliximab on histological changes in children with ulcerative colitis preliminary data. Journal of Crohn's and Colitis 2014;8:S249‐S250.
Additional references
Abraham 2009
Ardizzone 2011
-
- Ardizzone S, Cassinotti A, Duca P, Mazzali C, Penati C, Manes G, et al. Mucosal healing predicts late outcomes after the first course of corticosteroids for newly diagnosed ulcerative colitis. Clinical Gastroenterology and Hepatology 2011;9(6):483‐9. - PubMed
Baumgart 2007
-
- Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet 2007;369(9573):1641‐57. - PubMed
Bryant 2014
-
- Bryant RV, Winer S, Travis SP, Riddell RH. Systematic review: histological remission in inflammatory bowel disease. Is 'complete' remission the new treatment paradigm? An IOIBD initiative. Journal of Crohns and Colitis 2014;8(12):1582‐97. - PubMed
Cohen 1988
-
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd Edition. Hillsdale, NJ: Lawrence Erlbaum Associates, 1988.
Cohen 2002
-
- Cohen RD. Evolving medical therapies for ulcerative colitis. Current Gastroenterology Reports 2002;4(6):497‐505. - PubMed
Deyo 1991
-
- Deyo RA, Diehr P, Patrick DL. Reproducibility and responsiveness of health status measures. Statistics and strategies for evaluation. Controlled Clinical Trials 1991;12(4):S142‐58. - PubMed
Guyatt 1987
-
- Guyatt G, Walter S, Norman G. Measuring change over time: assessing the usefulness of evaluative instruments. Journal of Chronic Diseases 1987;40(2):171‐8. - PubMed
Landis 1977
-
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33(1):159–74. - PubMed
Pineton de Chambrun 2010
-
- Pineton de Chambrun G, Peyrin‐Biroulet L, Lemann M, Colombel JF. Clinical implications of mucosal healing for the management of IBD. Nature Reviews. Gastroenterology and Hepatology 2010;7(1):15‐29. - PubMed
Truelove 1955
Zou 2005
-
- Zou GY. Quantifying responsiveness of quality of life measures without an external criterion. Quality of Life Research 2005;14(6):1545‐52. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical