WITHDRAWN: Infant formulas containing hydrolysed protein for prevention of allergic disease and food allergy
- PMID: 28542713
- PMCID: PMC6481394
- DOI: 10.1002/14651858.CD003664.pub5
WITHDRAWN: Infant formulas containing hydrolysed protein for prevention of allergic disease and food allergy
Update in
-
Infant formulas containing hydrolysed protein for prevention of allergic disease.Cochrane Database Syst Rev. 2018 Oct 19;10(10):CD003664. doi: 10.1002/14651858.CD003664.pub6. Cochrane Database Syst Rev. 2018. PMID: 30338526 Free PMC article.
Abstract
Background: Allergy is common and may be associated with foods, including cow's milk formula (CMF). Formulas containing hydrolysed proteins have been used to treat infants with allergy. However, it is unclear whether hydrolysed formulas can be advocated for prevention of allergy in infants.
Objectives: To compare effects on allergy and food allergy when infants are fed a hydrolysed formula versus CMF or human breast milk. If hydrolysed formulas are effective, to determine what type of hydrolysed formula is most effective, including extensively or partially hydrolysed formula (EHF/PHF). To determine which infants at low or high risk of allergy and which infants receiving early, short-term or prolonged formula feeding may benefit from hydrolysed formulas.
Search methods: We used the standard search strategy of the Cochrane Neonatal Review Group supplemented by cross referencing of previous reviews and publications (updated August 2016).
Selection criteria: We searched for randomised and quasi-randomised trials that compared use of a hydrolysed formula versus human milk or CMF. Trials with ≥ 80% follow-up of participants were eligible for inclusion.
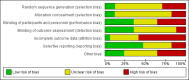
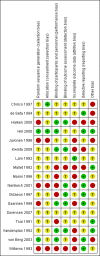
Data collection and analysis: We independently assessed eligibility of studies for inclusion, methodological quality and data extraction. Primary outcomes included clinical allergy, specific allergy and food allergy. We conducted meta-analysis using a fixed-effect (FE) model.
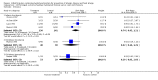
Main results: Two studies assessed the effect of three to four days' infant supplementation with an EHF whilst in hospital after birth versus pasteurised human milk feed. Results showed no difference in infant allergy or childhood cow's milk allergy (CMA). No eligible trials compared prolonged hydrolysed formula versus human milk feeding.Two studies assessed the effect of three to four days infant supplementation with an EHF versus a CMF. One large quasi-random study reported a reduction in infant CMA of borderline significance among low-risk infants (risk ratio (RR) 0.62, 95% confidence interval (CI) 0.38 to 1.00).Prolonged infant feeding with a hydrolysed formula compared with a CMF was associated with a reduction in infant allergy (eight studies, 2852 infants; FE RR 0.82, 95% CI 0.72 to 0.95; risk difference (RD) -0.04, 95% CI -0.08 to -0.01; number needed to treat for an additional beneficial outcome (NNTB) 25, 95% CI 12.5 to 100) and infant CMA (two studies, 405 infants; FE RR 0.38, 95% CI 0.16 to 0.86). We had substantial methodological concerns regarding studies and concerns regarding publication bias, as substantial numbers of studies including those in high-risk infants have not comprehensively reported allergy outcomes (GRADE quality of evidence 'very low').Prolonged infant feeding with a hydrolysed formula compared with a CMF was not associated with a difference in childhood allergy and led to no differences in specific allergy, including infant and childhood asthma, eczema and rhinitis and infant food allergy. Many of the analyses assessing specific allergy are underpowered.Subroup analyses showed that infant allergy was reduced in studies that enrolled infants at high risk of allergy who used a hydrolysed formula compared with a CMF; used a PHF compared with a CMF; used prolonged and exclusive feeding of a hydrolysed formula compared with a CMF; and used a partially hydrolysed whey formula compared with a CMF. Studies that enrolled infants at high risk of allergy; used a PHF compared with a CMF; used prolonged and exclusive feeding of a hydrolysed formula compared with a CMF; and used a partially hydrolysed whey formula compared with a CMF found a reduction in infant CMA.
Authors' conclusions: We found no evidence to support short-term or prolonged feeding with a hydrolysed formula compared with exclusive breast feeding for prevention of allergy. Very low-quality evidence indicates that short-term use of an EHF compared with a CMF may prevent infant CMA.In infants at high risk of allergy not exclusively breast fed, very low-quality evidence suggests that prolonged hydrolysed formula feeding compared with CMF feeding reduces infant allergy and infant CMA. Studies have found no difference in childhood allergy and no difference in specific allergy, including infant and childhood asthma, eczema and rhinitis and infant food allergy.Very low-quality evidence shows that prolonged use of a partially hydrolysed formula compared with a CMF for partial or exclusive feeding was associated with a reduction in infant allergy incidence and CMA incidence, and that prolonged use of an EHF versus a PHF reduces infant food allergy.
Conflict of interest statement
DO and JS have been invited speakers at industry‐organised scientific meetings. Neither has accepted an honorarium. LJ has no conflicts of interest to declare.
Figures










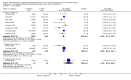























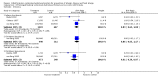











































Update of
-
Infant formulas containing hydrolysed protein for prevention of allergic disease and food allergy.Cochrane Database Syst Rev. 2017 Mar 15;3(3):CD003664. doi: 10.1002/14651858.CD003664.pub4. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2017 May 25;5:CD003664. doi: 10.1002/14651858.CD003664.pub5. PMID: 28293923 Free PMC article. Updated.
References
References to studies included in this review
-
- Chirico G, Gasparoni A, Ciardelli L, Amici M, Colombo A, Rondini G. Immunogenicity and antigenicity of a partially hydrolyzed cow's milk infant formula. Allergy 1997;52:82‐8. - PubMed
-
- Seta L, Siani P, Cirillo G, Gruttola M, Cimaduomo L, Coletta S. The prevention of allergic diseases with a hypoallergenic formula: a follow‐up at 24 months. The preliminary results. [Italian]. La Pediatria Medica e Chirurgica: Medical and Surgical Pediatrics 1994;16:251‐4. - PubMed
-
- Halken S, Hansen KS, Jacobsen HP, Estmann A, Faelling AE, Hansen LG, et al. Comparison of a partially hydrolyzed infant formula with two extensively hydrolyzed formulas for allergy prevention: a prospective, randomized study. Pediatric Allergy and Immunology 2000;11:149‐61. - PubMed
-
- Hill D. A report on the analysis of the Melbourn Atopy Cohort Study. A study designed to test the effectiveness of different formula types on the development of atopic symptoms and signs on a cohort of atopy‐at‐risk infants. Report at year 2. Nestec Internal Report.
- Hill DJ, Sporik R, Thorburn J, Hosking CS. The association of atopic dermatitis in infancy with immunoglobulin E food sensitization. Journal of Pediatrics 2000;137:475‐9. - PubMed
- Koplin J, Dharmage SC, Gurrin L, Osborne N, Tang ML, Lowe AJ, et al. Soy consumption is not a risk factor for peanut sensitization. Journal of Allergy and Clinical Immunology 2008;121:1455‐9. - PubMed
- Lowe A, Hosking C, Bennett C, Allen K, Carlin J, Dharmage S, et al. The Melbourne Atopy Cohort Study: a RCT of a partially hydrolysed whey formula for the prevention of allergic disease. Internal Medicine Journal. 2008; Vol. 38:A158.
- Lowe AJ, Abramson MJ, Hosking CS, Carlin JB, Bennett CM, Dharmage SC, et al. The temporal sequence of allergic sensitization and onset of infantile eczema. Clinical & Experimental Allergy 2007;37:536‐42. - PubMed
- Lowe AJ, Carlin JB, Bennett CM, Hosking CS, Allen KJ, Robertson CF, et al. Paracetamol use in early life and asthma: prospective birth cohort study. BMJ 2010;341:c4616. - PMC - PubMed
- Lowe AJ, Hosking CS, Bennett CM, Allen KJ, Axelrad C, Carlin JB, et al. Effect of a partially hydrolyzed whey infant formula at weaning on risk of allergic disease in high‐risk children: a randomized controlled trial. Journal of Allergy and Clinical Immunology 2011;128:360‐5, e4. - PubMed
- Stoney RM, Woods RK, Hosking CS, Hill DJ, Abramson MJ, Thien FC. Maternal breast milk long‐chain n‐3 fatty acids are associated with increased risk of atopy in breastfed infants. Clinical and Experimental Allergy 2004;34:194‐200. - PubMed
- Thomson JA, Widjaja C, Darmaputra AA, Lowe A, Matheson MC, Bennett CM, et al. Early childhood infections and immunisation and the development of allergic disease in particular asthma in a high‐risk cohort: a prospective study of allergy‐prone children from birth to six years. Pediatric Allergy and Immunology 2010;21:1076‐85. - PubMed
-
- Juvonen P, Mansson M, Andersson C, Jakobsson I. Allergy development and macromolecular absorption in infants with different feeding regimens during the first three days of life. A three‐year prospective follow‐up. Acta Paediatrica 1996;85:1047‐52. - PubMed
- Juvonen P, Mansson M, Jakobsson I. Does early diet have an effect on subsequent macromolecular absorption and serum IgE?. Journal of Pediatric Gastroenterology and Nutrition 1994;18:344‐9. - PubMed
- Juvonen P, Mansson M, Kjellman NI, Bjorksten B, Jakobsson I. Development of immunoglobulin G and immunoglobulin E antibodies to cow's milk proteins and ovalbumin after a temporary neonatal exposure to hydrolyzed and whole cow's milk proteins. Pediatric Allergy and Immunology 1999;10:191‐8. - PubMed
References to studies excluded from this review
-
- Agosti M, Pugni L, Ramenghi LA, Mosca F, Marini A. Hydrolysed proteins in preterm formula: influence on plasma aminoacids, blood fatty acids and insulinaemia. Acta Paediatrica Supplement 2003;91:34‐8. - PubMed
-
- Akimoto K, Saito H, Akasawa A, Iikura Y. [Preventative effect of a whey hydrolyzed formula (Nestle, NAN H.A.) on the development of allergic symptoms in infants]. [Japanese]. Arerugi ‐ Japanese Journal of Allergology 1997;46:1044‐51. - PubMed
-
- Arikan D, Alp H, Gozum S, Orbak Z, Cifci EK. Effectiveness of massage, sucrose solution, herbal tea or hydrolysed formula in the treatment of infantile colic. Journal of Clinical Nursing 2008;17:1754‐61. - PubMed
-
- Arshad SH, Bateman B, Matthews SM. Primary prevention of asthma and atopy during childhood by allergen avoidance in infancy: a randomised controlled study. Thorax 2003;58:489‐93. - PMC - PubMed
- Arshad SH, Matthews S, Gant C, Hide DW. Effect of allergen avoidance on development of allergic disorders in infancy. Lancet 1992;339:1493‐7. - PubMed
- Hide DW, Matthews S, Matthews L, Stevens M, Ridout S, Twiselton R, et al. Effect of allergen avoidance in infancy on allergic manifestations at age two years. Journal of Allergy and Clinical Immunology 1994;93:842‐6. - PubMed
- Hide DW, Matthews S, Tariq S, Arshad SH. Allergen avoidance in infancy and allergy at 4 years of age. Allergy 1996;51:89‐93. - PubMed
- Scott M, Kurukulaaratchy R, Roberts G, Clayton B, Matthews S, Arshad S. Primary prevention of asthma, outcomes at 17 year follow up. Allergy 2010;65; S92:315.
- Scott M, Roberts G, Kurukulaaratchy R, Clayton B, Matthews S, Arshad S. Primary prevention of eczema, outcomes at 17 year follow up. Allergy 2010;65; S92:314.
- Scott M, Roberts G, Kurukulaaratchy RJ, Matthews S, Nove A, Arshad SH. Multifaceted allergen avoidance during infancy reduces asthma during childhood with the effect persisting until age 18 years. Thorax 2012;67:1046‐51. - PubMed
-
- Barberi I, Salpietro DC, Catalioto G, Fulia F. Clinical trial of a new hypoallergenic formula for risk infants. 2nd World Congress of Perinatal Medicine. 1993.
References to ongoing studies
-
- Baldassarre M. Tolerance of an Extensively Hydrolyzed Protein Infant Formula Versus a Premature Infant Formula. ClinicalTrials.gov Identifier: NCT019871542013.
-
- Barber CM. Effects on Growth of an Extensively Hydrolyzed Formula Fed to Term. ClinicalTrials.gov Identifier: NCT009366372009.
-
- Moral T. Hydrolized Protein Formula for Premature Infants. ClinicalTrials.gov Identifier: NCT011564932010.
-
- Knip M. Early Dietary Intervention and Later Signs of Beta‐Cell Autoimmunity (EDIA). ClinicalTrials.gov Identifier: NCT01735123 2012. - PMC - PubMed
-
- Mennella JA. "Watch Your Baby Grow" Study (GRO). ClinicalTrials.gov Identifier: NCT017002052012.
Additional references
-
- Americal Academy of Pediatrics: Committee on Nutrition. Hypoallergenic infant formulas. Pediatrics 2000;106:346‐9. - PubMed
-
- Alexander DD, Cabana MD. Partially hydrolyzed 100% whey protein infant formula and reduced risk of atopic dermatitis: a meta‐analysis. Journal of Pediatric Gastroenterology and Nutrition 2010;50:422‐30. - PubMed
-
- Allen CW, Campbell DE, Kemp AS. Food allergy: is strict avoidance the only answer?. Pediatric Allergy and Immunology 2009;20:415‐22. - PubMed
-
- Arshad SH, Kurukulaaratchy RJ, Fenn M, Matthews S. Early life risk factors for current wheeze, asthma, and bronchial hyperresponsiveness at 10 years of age. Chest 2005;127:502‐8. - PubMed
-
- Bergmann RL, Bergmann KE, Lau‐Schadensdorf S, Luck W, Dannemann A, Bauer CP, et al. Atopic diseases in infancy. The German multicenter atopy study (MAS‐90). Pediatric Allergy and Immunology 1994;5(Suppl 6):19‐25. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

