Ibuprofen and diclofenac treatments reduce proliferation of pancreatic acinar cells upon inflammatory injury and mitogenic stimulation
- PMID: 28542719
- PMCID: PMC5758389
- DOI: 10.1111/bph.13867
Ibuprofen and diclofenac treatments reduce proliferation of pancreatic acinar cells upon inflammatory injury and mitogenic stimulation
Abstract
Background and purpose: Nonsteroidal anti-inflammatory drugs (NSAIDs) are administered to manage the pain typically found in patients suffering from pancreatitis. NSAIDs also display anti-proliferative activity against cancer cells; however, their effects on normal, untransformed cells are poorly understood. Here, we evaluated whether NSAIDs inhibit the proliferation of pancreatic acinar cells during the development of acute pancreatitis.
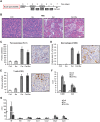
Experimental approach: The NSAIDs ibuprofen and diclofenac were administered to C57BL/6 mice after induction of pancreatitis with serial injections of cerulein. In addition, ibuprofen was administered concomitantly with 3,5,3-L-tri-iodothyronine (T3), which induces acinar cell proliferation in the absence of tissue inflammation. The development of pancreatic inflammation, acinar de-differentiation into metaplastic lesions and acinar proliferation were quantified by histochemical, biochemical and RT-PCR approaches.
Key results: Therapeutic ibuprofen treatment selectively reduced pancreatic infiltration of activated macrophages in vivo, and M1 macrophage polarization and pro-inflammatory cytokine expression both in vivo and in vitro. Reduced macrophage activation was accompanied by reduced acinar de-differentiation into acinar-to-ductal metaplasia. Acinar proliferation was significantly impaired in the presence of ibuprofen and diclofenac, as demonstrated at both the level of proliferation markers and expression of cell cycle regulators. Ibuprofen also reduced acinar cell proliferation induced by mitogenic stimulation with T3, a treatment that does not elicit pancreatic inflammation.
Conclusions and implications: Our study provides evidence that the NSAIDs ibuprofen and diclofenac inhibit pancreatic acinar cell division. This suggests that prolonged treatment with these NSAIDs may negatively affect the regeneration of the pancreas and further studies are needed to confirm these findings in a clinical setting.
Linked articles: This article is part of a themed section on Inventing New Therapies Without Reinventing the Wheel: The Power of Drug Repurposing. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v175.2/issuetoc.
© 2017 The British Pharmacological Society.
Figures





References
-
- Andrews J, Djakiew D, Krygier S, Andrews P (2002). Superior effectiveness of ibuprofen compared with other NSAIDs for reducing the survival of human prostate cancer cells. Cancer Chemother Pharmacol 50: 277–284. - PubMed
-
- Araki Y, Okamura S, Hussain SP, Nagashima M, He P, Shiseki M et al. (2003). Regulation of cyclooxygenase‐2 expression by the Wnt and ras pathways. Cancer Res 63: 728–734. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

