Tanshinone IIA ameliorates chronic arthritis in mice by modulating neutrophil activities
- PMID: 28542869
- PMCID: PMC5588760
- DOI: 10.1111/cei.12993
Tanshinone IIA ameliorates chronic arthritis in mice by modulating neutrophil activities
Abstract
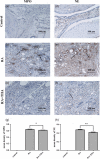
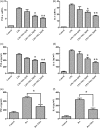
Rheumatoid arthritis (RA) is a chronic immune inflammatory disease mediated by the influx of immune cells into the synovial joint space. As Tanshinone IIA (TIIA) has potent anti-oxidant and anti-inflammatory activities, we used the adjuvant-induced arthritis (AA) murine model of RA to investigate the impact of TIIA on RA and immune cell activation. The anti-arthritic activity of TIIA was investigated in an adjuvant-induced arthritis model of RA in mice. Myeloperoxidase and neutrophil elastase expression levels were assessed in ankle joints by immunohistochemistry analysis. Immune cell infiltration was evaluated in air pouch experiments. Proinflammatory cytokines expression levels were determined by quantitative real-time polymerase chain reaction (PCR) and enzyme-linked immunosorbent assays. Neutrophil extracellular traps (NETs) were assessed by immunostaining and confocal microscopy. Treatment with TIIA alleviated cartilage erosion and neutrophil infiltration in the ankle joints of AA mice and reduced proinflammatory cytokine expression levels in sera. TIIA suppressed interleukin-6 and tumour necrosis factor-α expression and release in neutrophils and promoted neutrophil apoptosis. TIIA also inhibited the NET formation of neutrophils. Our findings demonstrated that TIIA can ameliorate RA effectively by targeting neutrophils, indicating that TIIA may act as a potential therapeutic for RA.
Keywords: arthritis; inflammation; neutrophil; tanshinone IIA.
© 2017 British Society for Immunology.
Figures




Similar articles
-
Triptolide inhibits the inflammatory activities of neutrophils to ameliorate chronic arthritis.Mol Immunol. 2018 Sep;101:210-220. doi: 10.1016/j.molimm.2018.06.012. Epub 2018 Jul 5. Mol Immunol. 2018. PMID: 30007231
-
Anti-nociceptive effects of Tanshinone IIA (TIIA) in a rat model of complete Freund's adjuvant (CFA)-induced inflammatory pain.Brain Res Bull. 2012 Sep 1;88(6):581-8. doi: 10.1016/j.brainresbull.2012.06.002. Epub 2012 Jun 13. Brain Res Bull. 2012. PMID: 22705002
-
Andrographolide Ameliorates Rheumatoid Arthritis by Regulating the Apoptosis-NETosis Balance of Neutrophils.Int J Mol Sci. 2019 Oct 11;20(20):5035. doi: 10.3390/ijms20205035. Int J Mol Sci. 2019. PMID: 31614480 Free PMC article.
-
Neutrophil Function in an Inflammatory Milieu of Rheumatoid Arthritis.J Immunol Res. 2018 Dec 3;2018:8549329. doi: 10.1155/2018/8549329. eCollection 2018. J Immunol Res. 2018. PMID: 30622982 Free PMC article. Review.
-
Neutrophil extracellular traps: Potential targets for the treatment of rheumatoid arthritis with traditional Chinese medicine and natural products.Phytother Res. 2024 Nov;38(11):5067-5087. doi: 10.1002/ptr.8311. Epub 2024 Aug 6. Phytother Res. 2024. PMID: 39105461 Review.
Cited by
-
Expanding the therapeutic potential of Salvia miltiorrhiza: a review of its pharmacological applications in musculoskeletal diseases.Front Pharmacol. 2023 Dec 5;14:1276038. doi: 10.3389/fphar.2023.1276038. eCollection 2023. Front Pharmacol. 2023. PMID: 38116081 Free PMC article. Review.
-
Tanshinone IIA promotes the apoptosis of fibroblast-like synoviocytes in rheumatoid arthritis by up-regulating lncRNA GAS5.Biosci Rep. 2018 Oct 5;38(5):BSR20180626. doi: 10.1042/BSR20180626. Print 2018 Oct 31. Biosci Rep. 2018. PMID: 30232236 Free PMC article.
-
Tanshinone IIA inhibits osteoclastogenesis in rheumatoid arthritis via LDHC-regulated ROS generation.Chin Med. 2023 May 15;18(1):54. doi: 10.1186/s13020-023-00765-1. Chin Med. 2023. PMID: 37189204 Free PMC article.
-
Tanshinone IIA alleviates brain damage in a mouse model of neuromyelitis optica spectrum disorder by inducing neutrophil apoptosis.J Neuroinflammation. 2020 Jun 25;17(1):198. doi: 10.1186/s12974-020-01874-6. J Neuroinflammation. 2020. PMID: 32586353 Free PMC article.
-
Activities and Molecular Mechanisms of Diterpenes, Diterpenoids, and Their Derivatives in Rheumatoid Arthritis.Evid Based Complement Alternat Med. 2022 Mar 25;2022:4787643. doi: 10.1155/2022/4787643. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35368757 Free PMC article. Review.
References
-
- Cascão R, Rosário HS, Souto‐Carneiro MM, Fonseca JE. Neutrophils in rheumatoid arthritis: more than simple final effectors. Autoimmun Rev 2010; 9:531–5. - PubMed
-
- Wright HL, Moots RJ, Edwards SW. The multifactorial role of neutrophils in rheumatoid arthritis. Nat Rev Rheumatol 2014; 10:593–601. - PubMed
-
- Eyles JL, Roberts AW, Metcalf D, Wicks IP. Granulocyte colony‐stimulating factor and neutrophils–forgotten mediators of inflammatory disease. Nat Clin Pract Rheumatol 2006; 2:500–10. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

