The polysialic acid mimetics idarubicin and irinotecan stimulate neuronal survival and neurite outgrowth and signal via protein kinase C
- PMID: 28542923
- PMCID: PMC5539918
- DOI: 10.1111/jnc.14076
The polysialic acid mimetics idarubicin and irinotecan stimulate neuronal survival and neurite outgrowth and signal via protein kinase C
Abstract
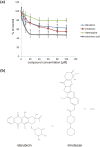
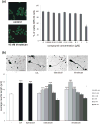
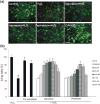
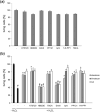
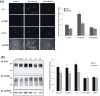
Polysialic acid (PSA) is a large, negatively charged, linear homopolymer of alpha2-8-linked sialic acid residues. It is generated by two polysialyltransferases and attached to N- and/or O-linked glycans, and its main carrier is the neural cell adhesion molecule (NCAM). PSA controls the development and regeneration of the nervous system by enhancing cell migration, axon pathfinding, synaptic targeting, synaptic plasticity, by regulating the differentiation of progenitor cells and by modulating cell-cell and cell-matrix adhesions. In the adult, PSA plays a role in the immune system, and PSA mimetics promote functional recovery after nervous system injury. In search for novel small molecule mimetics of PSA that are applicable for therapy, we identified idarubicin, an antineoplastic anthracycline, and irinotecan, an antineoplastic agent of the topoisomerase I inhibitor class, as PSA mimetics using a competition enzyme-linked immunosorbent assay. Idarubicin and irinotecan compete with the PSA-mimicking peptide and colominic acid, the bacterial analog of PSA, for binding to the PSA-specific monoclonal antibody 735. Idarubicin and irinotecan stimulate neurite outgrowth and survival of cultured cerebellar neurons after oxidative stress via protein kinase C and Erk1/2 in a similar manner as colominic acid, whereas Fyn, casein kinase II and the phosphatase and tensin homolog are only involved in idarubicin and irinotecan-stimulated neurite outgrowth. These novel results show that the structure and function of PSA can be mimicked by the small organic compounds irinotecan and idarubicin which trigger the same signaling cascades as PSA, thus introducing the possibility of retargeting these drugs to treat nervous system injuries.
Keywords: cell survival; idarubicin; irinotecan; neurite outgrowth; polysialic acid.
© 2017 International Society for Neurochemistry.
Figures

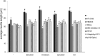
Similar articles
-
Vinorelbine and epirubicin share common features with polysialic acid and modulate neuronal and glial functions.J Neurochem. 2016 Jan;136(1):48-62. doi: 10.1111/jnc.13383. Epub 2015 Nov 6. J Neurochem. 2016. PMID: 26443186 Free PMC article.
-
Nonyloxytryptamine mimics polysialic acid and modulates neuronal and glial functions in cell culture.J Neurochem. 2014 Jan;128(1):88-100. doi: 10.1111/jnc.12408. Epub 2013 Sep 23. J Neurochem. 2014. PMID: 23957498
-
PSA-NCAM in mammalian structural plasticity and neurogenesis.Prog Neurobiol. 2006 Oct;80(3):129-64. doi: 10.1016/j.pneurobio.2006.08.003. Epub 2006 Oct 9. Prog Neurobiol. 2006. PMID: 17029752 Review.
-
Regulation of neural cell adhesion molecule polysialylation state by cell-cell contact and protein kinase C delta.J Neurosci Res. 2000 Sep 15;61(6):636-45. doi: 10.1002/1097-4547(20000915)61:6<636::AID-JNR7>3.0.CO;2-F. J Neurosci Res. 2000. PMID: 10972960
-
Contribution of the neural cell adhesion molecule to neuronal and synaptic plasticity.Rev Neurosci. 2001;12(4):297-310. doi: 10.1515/revneuro.2001.12.4.297. Rev Neurosci. 2001. PMID: 11783716 Review.
Cited by
-
New high-density fermentation method for producing high molecular weight polysialic acid based on the combination fermentation strategy.Appl Microbiol Biotechnol. 2022 Apr;106(7):2381-2391. doi: 10.1007/s00253-022-11874-6. Epub 2022 Mar 12. Appl Microbiol Biotechnol. 2022. PMID: 35278115
-
Application of Antibodies to Neuronally Expressed Nogo-A Increases Neuronal Survival and Neurite Outgrowth.Int J Mol Sci. 2020 Jul 30;21(15):5417. doi: 10.3390/ijms21155417. Int J Mol Sci. 2020. PMID: 32751444 Free PMC article.
-
Enhanced Neuronal Survival and Neurite Outgrowth Triggered by Novel Small Organic Compounds Mimicking the LewisX Glycan.Mol Neurobiol. 2018 Oct;55(10):8203-8215. doi: 10.1007/s12035-018-0953-8. Epub 2018 Mar 8. Mol Neurobiol. 2018. PMID: 29520715 Free PMC article.
-
Functions of Small Organic Compounds that Mimic the HNK-1 Glycan.Int J Mol Sci. 2020 Sep 24;21(19):7018. doi: 10.3390/ijms21197018. Int J Mol Sci. 2020. PMID: 32987628 Free PMC article.
-
Neural glycomics: the sweet side of nervous system functions.Cell Mol Life Sci. 2021 Jan;78(1):93-116. doi: 10.1007/s00018-020-03578-9. Epub 2020 Jul 1. Cell Mol Life Sci. 2021. PMID: 32613283 Free PMC article. Review.
References
-
- Bonfanti L. PSA-NCAM in mammalian structural plasticity and neurogenesis. Prog Neurobiol. 2006;80:129–164. - PubMed
-
- Brezun JM, Daszuta A. Serotonergic reinnervation reverses lesion-induced decreases in PSA-NCAM labeling and proliferation of hippocampal cells in adult rats. Hippocampus. 2000;10:37–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

