Compensatory and decompensatory alterations in cardiomyocyte Ca2+ dynamics in hearts with diastolic dysfunction following aortic banding
- PMID: 28542952
- PMCID: PMC5471387
- DOI: 10.1113/JP273879
Compensatory and decompensatory alterations in cardiomyocyte Ca2+ dynamics in hearts with diastolic dysfunction following aortic banding
Abstract
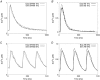
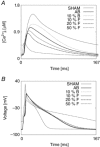
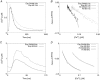
Key points: At the cellular level cardiac hypertrophy causes remodelling, leading to changes in ionic channel, pump and exchanger densities and kinetics. Previous studies have focused on quantifying changes in channels, pumps and exchangers without quantitatively linking these changes with emergent cellular scale functionality. Two biophysical cardiac cell models were created, parameterized and validated and are able to simulate electrophysiology and calcium dynamics in myocytes from control sham operated rats and aortic-banded rats exhibiting diastolic dysfunction. The contribution of each ionic pathway to the calcium kinetics was calculated, identifying the L-type Ca2+ channel and sarco/endoplasmic reticulum Ca2+ ATPase as the principal regulators of systolic and diastolic Ca2+ , respectively. Results show that the ability to dynamically change systolic Ca2+ , through changes in expression of key Ca2+ modelling protein densities, is drastically reduced following the aortic banding procedure; however the cells are able to compensate Ca2+ homeostasis in an efficient way to minimize systolic dysfunction.
Abstract: Elevated left ventricular afterload leads to myocardial hypertrophy, diastolic dysfunction, cellular remodelling and compromised calcium dynamics. At the cellular scale this remodelling of the ionic channels, pumps and exchangers gives rise to changes in the Ca2+ transient. However, the relative roles of the underlying subcellular processes and the positive or negative impact of each remodelling mechanism are not fully understood. Biophysical cardiac cell models were created to simulate electrophysiology and calcium dynamics in myocytes from control rats (SHAM) and aortic-banded rats exhibiting diastolic dysfunction. The model parameters and framework were validated and the fitted parameters demonstrated to be unique for explaining our experimental data. The contribution of each ionic pathway to the calcium kinetics was calculated, identifying the L-type Ca2+ channel (LCC) and the sarco/endoplasmic reticulum Ca2+ -ATPase (SERCA) as the principal regulators of systolic and diastolic Ca2+ , respectively. In the aortic banding model, the sensitivity of systolic Ca2+ to LCC density and diastolic Ca2+ to SERCA density decreased by 16-fold and increased by 23%, respectively, relative to the SHAM model. The energy cost of ionic homeostasis is maintained across the two models. The models predict that changes in ionic pathway densities in compensated aortic banding rats maintain Ca2+ function and efficiency. The ability to dynamically alter systolic function is significantly diminished, while the capacity to maintain diastolic Ca2+ is moderately increased.
Keywords: calcium dynamics; cardiac electrophysiology; cell biology; computational modelling; diastolic dysfunction; gene expression; hypertrophy; rat.
© 2017 The Authors. The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Figures












References
-
- Aeschbacher BC, Hutter D, Fuhrer J, Weidmann P, Delacrétaz E & Allemann Y (2001). Diastolic dysfunction precedes myocardial hypertrophy in the development of hypertension. Am J Hypertens 14, 106–113. - PubMed
-
- Amin AS, Tan HL & Wilde AAM (2010). Cardiac ion channels in health and disease. Hear Rhythm 7, 117–126. - PubMed
-
- Bailey BA & Houser SR (1992). Calcium transients in feline left ventricular myocytes with hypertrophy induced by slow progressive pressure overload. J Mol Cell Cardiol 24, 365–373. - PubMed
-
- Balke CW & Shorofsky SR (1998). Alterations in calcium handling in cardiac hypertrophy and heart failure. Cardiovasc Res 37, 290–299. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

