Prenatal environmental tobacco smoke exposure increases allergic asthma risk with methylation changes in mice
- PMID: 28543436
- PMCID: PMC5513771
- DOI: 10.1002/em.22097
Prenatal environmental tobacco smoke exposure increases allergic asthma risk with methylation changes in mice
Abstract
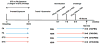
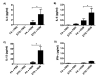
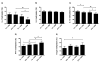
Allergic asthma remains an inadequately understood disease. In utero exposure to environmental tobacco smoke (ETS) has been identified as an environmental exposure that can increase an individual's asthma risk. To improve our understanding of asthma onset and development, we examined the effect of in utero ETS exposure on allergic disease susceptibility in an asthmatic phenotype using a house dust mite (HDM) allergen-induced murine model. Pregnant C57BL/6 mice were exposed to either filtered air or ETS during gestation, and their offspring were further exposed to HDM at 6-7 weeks old to induce allergic inflammation. Methylation in the promoter regions of allergic inflammation-related genes and genomic DNA was quantified. Exposure to HDM resulted in the onset of allergic lung inflammation, with an increased presence of inflammatory cells, Th2 cytokines (IL-4, IL-5, and IL-13), and airway remodeling. These asthmatic phenotypes were significantly enhanced when the mice had been exposed to in utero ETS. Furthermore, prenatal ETS exposure and subsequent HDM (ETS/HDM)-induced asthmatic phenotypes agree with methylation changes in the selected asthma-related genes, including IL-4, IL-5, IL-13, INF-γ, and FOXP3. Global DNA methylation was significantly lower in ETS/HDM-exposed mice than that of controls, which coincides with the results observed in lung, spleen, and blood DNAs. Prenatal ETS exposure resulted in a severe increase in allergic inflammatory responses after an HDM challenge, with corresponding methylation changes. Prenatal ETS exposure may influence developmental plasticity and result in altered epigenetic programming, leading to an increased susceptibility to asthma. Environ. Mol. Mutagen. 58:423-433, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: HDM murine model; allergic asthma; environmental tobacco smoke; in utero; methylation.
© 2017 Wiley Periodicals, Inc.
Conflict of interest statement
Figures
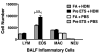

Similar articles
-
Prenatal tobacco smoke exposure predisposes offspring mice to exacerbated allergic airway inflammation associated with altered innate effector function.Part Fibre Toxicol. 2017 Aug 22;14(1):30. doi: 10.1186/s12989-017-0212-6. Part Fibre Toxicol. 2017. PMID: 28830530 Free PMC article.
-
Alterations in DNA methylation and airway hyperreactivity in response to in utero exposure to environmental tobacco smoke.Inhal Toxicol. 2015;27(13):724-30. doi: 10.3109/08958378.2015.1104402. Epub 2015 Nov 2. Inhal Toxicol. 2015. PMID: 26525176 Free PMC article.
-
In Utero Cigarette Smoke Affects Allergic Airway Disease But Does Not Alter the Lung Methylome.PLoS One. 2015 Dec 7;10(12):e0144087. doi: 10.1371/journal.pone.0144087. eCollection 2015. PLoS One. 2015. PMID: 26642056 Free PMC article.
-
Prevention of allergic disease in childhood: clinical and epidemiological aspects of primary and secondary allergy prevention.Pediatr Allergy Immunol. 2004 Jun;15 Suppl 16:4-5, 9-32. doi: 10.1111/j.1399-3038.2004.0148b.x. Pediatr Allergy Immunol. 2004. PMID: 15125698 Review.
-
Environmental tobacco smoke (ETS) and respiratory health in children.Eur J Pediatr. 2009 Aug;168(8):897-905. doi: 10.1007/s00431-009-0967-3. Epub 2009 Mar 20. Eur J Pediatr. 2009. PMID: 19301035 Review.
Cited by
-
Interactions between environmental pollutants and genetic susceptibility in asthma risk.Curr Opin Immunol. 2019 Oct;60:156-162. doi: 10.1016/j.coi.2019.07.010. Epub 2019 Aug 28. Curr Opin Immunol. 2019. PMID: 31470287 Free PMC article. Review.
-
Prenatal and Postnatal Cigarette Smoke Exposure Is Associated With Increased Risk of Exacerbated Allergic Airway Immune Responses: A Preclinical Mouse Model.Front Immunol. 2021 Dec 23;12:797376. doi: 10.3389/fimmu.2021.797376. eCollection 2021. Front Immunol. 2021. PMID: 35003121 Free PMC article.
-
Familial confounding of the association between maternal smoking in pregnancy and autism spectrum disorder in offspring.Autism Res. 2020 Jan;13(1):134-144. doi: 10.1002/aur.2196. Epub 2019 Aug 29. Autism Res. 2020. PMID: 31464107 Free PMC article.
-
Air pollution-induced epigenetic changes: disease development and a possible link with hypersensitivity pneumonitis.Environ Sci Pollut Res Int. 2021 Oct;28(40):55981-56002. doi: 10.1007/s11356-021-16056-x. Epub 2021 Sep 8. Environ Sci Pollut Res Int. 2021. PMID: 34498177 Free PMC article. Review.
-
Mmp12 Is Upregulated by in utero Second-Hand Smoke Exposures and Is a Key Factor Contributing to Aggravated Lung Responses in Adult Emphysema, Asthma, and Lung Cancer Mouse Models.Front Physiol. 2021 Nov 29;12:704401. doi: 10.3389/fphys.2021.704401. eCollection 2021. Front Physiol. 2021. PMID: 34912233 Free PMC article.
References
-
- Bloemen K, Verstraelen S, Van Den Heuvel R, Witters H, Nelissen I, Schoeters G. The allergic cascade: review of the most important molecules in the asthmatic lung. Immunol Lett. 2007;113(1):6–18. - PubMed
-
- Brand S, Kesper DA, Teich R, Kilic-Niebergall E, Pinkenburg O, Bothur E, Lohoff M, Garn H, Pfefferle PI, Renz H. DNA methylation of TH1/TH2 cytokine genes affects sensitization and progress of experimental asthma. J Allergy Clin Immunol. 2012;129(6):1602–1610 e1606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

