SMAD3 expression and regulation of fibroplasia in vocal fold injury
- PMID: 28543554
- PMCID: PMC5568935
- DOI: 10.1002/lary.26648
SMAD3 expression and regulation of fibroplasia in vocal fold injury
Abstract
Objective: Recent reports highlight the efficacy of small interfering RNA (siRNA) targeting SMAD3 to regulate transforming growth factor β (TGF-β)-mediated fibroplasia in vocal fold fibroblasts. The current study sought to investigate SMAD3 expression during wound healing in vivo and quantify the downstream transcriptional events associated with SMAD3 knockdown in vitro.
Study design: In vivo and in vitro.
Methods: Unilateral vocal fold injury was created in a rabbit model. SMAD3 and SMAD7 mRNA expression was quantified at 1 hour and 1, 3, 7, 14, 30, 60, and 90 days following injury. In vitro, multi-gene analysis technology was employed in our immortalized human vocal-fold fibroblast cell line following TGF-β1 stimulation ± SMAD3 knockdown across time points.
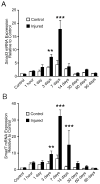
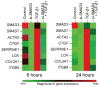
Results: SMAD3 mRNA expression increased following injury; upregulation was significant at 3 and 7 days compared to control (both P < 0.001). SMAD7 mRNA was also upregulated at 3, 7, and 14 days (P = 0.02, P < 0.001, and P < 0.001, respectively). In vitro, SMAD3 knockdown reduced the expression of multiple profibrotic, TGF-β signaling, and extracellular matrix metabolism genes at 6 and 24 hours following TGF-β1 stimulation.
Conclusion: Cumulatively, these data support SMAD3 as a potential master regulator of TGF-β-mediated fibrosis. SMAD3 transcription peaked 7 days following injury. Multi-gene analysis indicated that the therapeutic effectiveness of SMAD3 knockdown may be related to regulation of downstream mediators of fibroplasia and altered TGF-β signaling.
Level of evidence: NA. Laryngoscope, 127:E308-E316, 2017.
Keywords: PCR array; SMAD3; Vocal fold; fibrosis; siRNA; transforming growth factor-β.
© 2017 The American Laryngological, Rhinological and Otological Society, Inc.
Conflict of interest statement
The authors have no financial disclosures or conflicts of interest.
Figures
Similar articles
-
NR4A1 is an endogenous inhibitor of vocal fold fibrosis.Laryngoscope. 2017 Sep;127(9):E317-E323. doi: 10.1002/lary.26678. Epub 2017 Jun 5. Laryngoscope. 2017. PMID: 28581197 Free PMC article.
-
The role of Smad3 in the fibrotic phenotype in human vocal fold fibroblasts.Laryngoscope. 2016 May;126(5):1151-6. doi: 10.1002/lary.25673. Epub 2015 Sep 30. Laryngoscope. 2016. PMID: 26422444 Free PMC article.
-
Expression of tenascin-C in a rat vocal fold injury model and its regulation of fibroblasts.Laryngoscope. 2018 Sep;128(9):E316-E322. doi: 10.1002/lary.27164. Epub 2018 Mar 23. Laryngoscope. 2018. PMID: 29572861
-
[Transforming growth factor-β and renal fibrosis].Sheng Li Xue Bao. 2018 Dec 25;70(6):612-622. Sheng Li Xue Bao. 2018. PMID: 30560270 Review. Chinese.
-
Central role of dysregulation of TGF-β/Smad in CKD progression and potential targets of its treatment.Biomed Pharmacother. 2018 May;101:670-681. doi: 10.1016/j.biopha.2018.02.090. Epub 2018 Mar 22. Biomed Pharmacother. 2018. PMID: 29518614 Review.
Cited by
-
Preclinical Vocal Fold and Airway Injury Models: A Scoping Review.Laryngoscope Investig Otolaryngol. 2025 Jul 31;10(4):e70173. doi: 10.1002/lio2.70173. eCollection 2025 Aug. Laryngoscope Investig Otolaryngol. 2025. PMID: 40746632 Free PMC article. Review.
-
Implementing Efficient Peptoid-Mediated Delivery of RNA-Based Therapeutics to the Vocal Folds.Laryngoscope Investig Otolaryngol. 2019 Oct 22;4(6):640-644. doi: 10.1002/lio2.310. eCollection 2019 Dec. Laryngoscope Investig Otolaryngol. 2019. PMID: 31890882 Free PMC article.
-
Concurrent YAP/TAZ and SMAD signaling mediate vocal fold fibrosis.Sci Rep. 2021 Jun 29;11(1):13484. doi: 10.1038/s41598-021-92871-z. Sci Rep. 2021. PMID: 34188130 Free PMC article.
-
Nanoparticle delivery of RNA-based therapeutics to alter the vocal fold tissue response to injury.Laryngoscope. 2018 May;128(5):E178-E183. doi: 10.1002/lary.27047. Epub 2017 Dec 14. Laryngoscope. 2018. PMID: 29238989 Free PMC article.
-
SOCS3 Silencing Promotes Activation of Vocal Fold Fibroblasts via JAK2/STAT3 Signaling Pathway.Inflammation. 2023 Aug;46(4):1318-1331. doi: 10.1007/s10753-023-01810-9. Epub 2023 May 8. Inflammation. 2023. PMID: 37154979
References
-
- Benninger MS, Alessi D, Archer S, et al. Vocal fold scarring: current concepts and management. Otolaryngol Head Neck Surg. 1996;115:474–482. - PubMed
-
- Hirano S, Minamiguchi S, Yamashita M, Ohno T, Kanemaru S, Kitamura M. Histologic characterization of human scarred vocal folds. J Voice. 2009;23:399–407. - PubMed
-
- Liu W, Wang DR, Cao YL. TGF-beta: a fibrotic factor in wound scarring and a potential target for anti-scarring gene therapy. Curr Gene Ther. 2004;4:123–136. - PubMed
-
- Grande JP. Role of transforming growth factor-beta in tissue injury and repair. Proc Soc Exp Biol Med. 1997;214:27–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

