TGR5 contributes to hepatic cystogenesis in rodents with polycystic liver diseases through cyclic adenosine monophosphate/Gαs signaling
- PMID: 28543567
- PMCID: PMC5605412
- DOI: 10.1002/hep.29284
TGR5 contributes to hepatic cystogenesis in rodents with polycystic liver diseases through cyclic adenosine monophosphate/Gαs signaling
Abstract
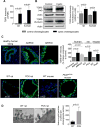
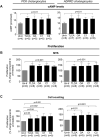
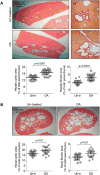
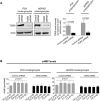
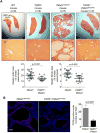
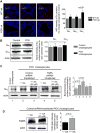
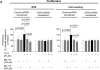
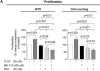
Hepatic cystogenesis in polycystic liver disease is associated with increased levels of cyclic adenosine monophosphate (cAMP) in cholangiocytes lining liver cysts. Takeda G protein receptor 5 (TGR5), a G protein-coupled bile acid receptor, is linked to cAMP and expressed in cholangiocytes. Therefore, we hypothesized that TGR5 might contribute to disease progression. We examined expression of TGR5 and Gα proteins in cultured cholangiocytes and in livers of animal models and humans with polycystic liver disease. In vitro, we assessed cholangiocyte proliferation, cAMP levels, and cyst growth in response to (1) TGR5 agonists (taurolithocholic acid, oleanolic acid [OA], and two synthetic compounds), (2) a novel TGR5 antagonist (m-tolyl 5-chloro-2-[ethylsulfonyl] pyrimidine-4-carboxylate [SBI-115]), and (3) a combination of SBI-115 and pasireotide, a somatostatin receptor analogue. In vivo, we examined hepatic cystogenesis in OA-treated polycystic kidney rats and after genetic elimination of TGR5 in double mutant TGR5-/- ;Pkhd1del2/del2 mice. Compared to control, expression of TGR5 and Gαs (but not Gαi and Gαq ) proteins was increased 2-fold to 3-fold in cystic cholangiocytes in vitro and in vivo. In vitro, TGR5 stimulation enhanced cAMP production, cell proliferation, and cyst growth by ∼40%; these effects were abolished after TGR5 reduction by short hairpin RNA. OA increased cystogenesis in polycystic kidney rats by 35%; in contrast, hepatic cystic areas were decreased by 45% in TGR5-deficient TGR5-/- ;Pkhd1del2/del2 mice. TGR5 expression and its colocalization with Gαs were increased ∼2-fold upon OA treatment. Levels of cAMP, cell proliferation, and cyst growth in vitro were decreased by ∼30% in cystic cholangiocytes after treatment with SBI-115 alone and by ∼50% when SBI-115 was combined with pasireotide.
Conclusion: TGR5 contributes to hepatic cystogenesis by increasing cAMP and enhancing cholangiocyte proliferation; our data suggest that a TGR5 antagonist alone or concurrently with somatostatin receptor agonists represents a potential therapeutic approach in polycystic liver disease. (Hepatology 2017;66:1197-1218).
© 2017 by the American Association for the Study of Liver Diseases.
Conflict of interest statement
Figures










Similar articles
-
Combination of a Histone Deacetylase 6 Inhibitor and a Somatostatin Receptor Agonist Synergistically Reduces Hepatorenal Cystogenesis in an Animal Model of Polycystic Liver Disease.Am J Pathol. 2018 Apr;188(4):981-994. doi: 10.1016/j.ajpath.2017.12.016. Epub 2018 Jan 31. Am J Pathol. 2018. PMID: 29366679 Free PMC article.
-
TGR5 in the Cholangiociliopathies.Dig Dis. 2015;33(3):420-5. doi: 10.1159/000371696. Epub 2015 May 27. Dig Dis. 2015. PMID: 26045278 Free PMC article.
-
Targeting of a Bile Acid Receptor, Takeda G Protein Receptor 5 [TGR5], by a Novel First-in-Class Competitive Antagonist, SBI-364, Diminishes Hepatic and Renal Cystogenesis in Polycystic Liver and Kidney Disease.ACS Pharmacol Transl Sci. 2025 Jun 30;8(7):1950-1964. doi: 10.1021/acsptsci.4c00701. eCollection 2025 Jul 11. ACS Pharmacol Transl Sci. 2025. PMID: 40672683
-
Bile acid receptors in the biliary tree: TGR5 in physiology and disease.Biochim Biophys Acta Mol Basis Dis. 2018 Apr;1864(4 Pt B):1319-1325. doi: 10.1016/j.bbadis.2017.08.021. Epub 2017 Aug 25. Biochim Biophys Acta Mol Basis Dis. 2018. PMID: 28844960 Review.
-
Role of the G Protein-Coupled Bile Acid Receptor TGR5 in Liver Damage.Dig Dis. 2017;35(3):235-240. doi: 10.1159/000450917. Epub 2017 Mar 1. Dig Dis. 2017. PMID: 28249265 Review.
Cited by
-
Identification of the Role of TGR5 in the Regulation of Leydig Cell Homeostasis.Int J Mol Sci. 2022 Dec 6;23(23):15398. doi: 10.3390/ijms232315398. Int J Mol Sci. 2022. PMID: 36499726 Free PMC article.
-
Therapeutic potential of oleanolic acid in liver diseases.Naunyn Schmiedebergs Arch Pharmacol. 2024 Jul;397(7):4537-4554. doi: 10.1007/s00210-024-02959-2. Epub 2024 Jan 31. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38294504 Review.
-
Proanthocyanidin B2 derived metabolites may be ligands for bile acid receptors S1PR2, PXR and CAR: an in silico approach.J Biomol Struct Dyn. 2024 May;42(8):4249-4262. doi: 10.1080/07391102.2023.2224886. Epub 2023 Jun 20. J Biomol Struct Dyn. 2024. PMID: 37340688 Free PMC article.
-
Intrahepatic cystic biliary dilatation constitutes a significant prognostic factor in patients with primary sclerosing cholangitis.Eur Radiol. 2019 Mar;29(3):1460-1468. doi: 10.1007/s00330-018-5697-3. Epub 2018 Aug 29. Eur Radiol. 2019. PMID: 30159619
-
Smooth muscle contractile responses to bile acids in mouse ileum require TGR5 but not ASBT.Front Neurol. 2024 Apr 24;15:1334319. doi: 10.3389/fneur.2024.1334319. eCollection 2024. Front Neurol. 2024. PMID: 38721114 Free PMC article.
References
-
- Wills ES, Roepman R, Drenth JP. Polycystic liver disease: ductal plate malformation and the primary cilium. Trends in molecular medicine. 2014 - PubMed
-
- Masyuk TV, Masyuk AI, Torres VE, et al. Octreotide inhibits hepatic cystogenesis in a rodent model of polycystic liver disease by reducing cholangiocyte adenosine 3',5'-cyclic monophosphate. Gastroenterology. 2007;132:1104–16. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

