Neuromuscular junctions (NMJs): ultrastructural analysis and nicotinic acetylcholine receptor (nAChR) subunit mRNA expression in offspring subjected to protein restriction throughout pregnancy
- PMID: 28543723
- PMCID: PMC5485363
- DOI: 10.1111/iep.12229
Neuromuscular junctions (NMJs): ultrastructural analysis and nicotinic acetylcholine receptor (nAChR) subunit mRNA expression in offspring subjected to protein restriction throughout pregnancy
Abstract
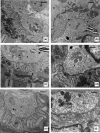
Protein restriction during gestation can alter the skeletal muscle phenotype of offspring; however, little is known with regard to whether this also affects the neuromuscular junction (NMJ), as muscle phenotype maintenance depends upon NMJ functional integrity. This study aimed to evaluate the effects of a low protein (6%) intake by dams throughout gestation on male offspring NMJ morphology and nicotinic acetylcholine receptor (nAChR) α1, γ and ε subunit expression in the soleus (SOL) and extensor digitorum longus (EDL) muscles. Four groups of male Wistar offspring rats were studied. The offspring of dams fed low-protein (6% protein, LP) and normal protein (17% protein, NP) diets were evaluated at 30 and 120 days of age, and the SOL and EDL muscles were collected for analysis. Morphological studies using transmission electron microscopy revealed that only SOL NMJs were affected in 30-day-old offspring in the LP group compared with the NP group. SOL NMJs exhibited fewer synaptic folds, the postsynaptic membranes were smooth and myelin figures were also frequently observed in the terminal axons. With regard to the expression of mRNAs encoding nAChR subunits, only 30-day-old LP offspring EDL muscles exhibited reduced α, γ and ε subunit expression compared with the NP group. In conclusion, our results demonstrate that a low-protein diet (6%) imposed throughout pregnancy impairs the expression of mRNAs encoding the nAChR α, γ and ε subunits in EDL NMJs and promotes morphological changes in SOL NMJs of 30-day-old offspring, indicating specific differences among muscle types following long-term maternal protein restriction.
Keywords: neuromuscular junction; pregnancy; protein restriction; skeletal muscle.
© 2017 The Authors. International Journal of Experimental Pathology © 2017 International Journal of Experimental Pathology.
Figures


References
-
- Andonian M.H. & Fahim M.A. (1988) Endurance exercise alters the morphology of fast‐ and slow‐twitch rat neuromuscular junctions. Int. J. Sports Med. 9, 218–223. - PubMed
-
- Aragão R.S., Guzmán‐Quevedo O., Pérez‐García G., Manhães‐de‐Castro R. & Bolaños‐Jiménez F. (2014) Maternal protein restriction impairs the transcriptional metabolic flexibility of skeletal muscle in adult rat offspring . Br. J. Nutr. 3, 328–337. - PubMed
-
- Barker D. & Ip M.C. (1966) Sprouting and degeneration of mammalian motor axons in normal and de‐afferented skeletal muscle. Proc. R. Soc. Lond. B Biol. Sci. 163, 538–554. - PubMed
-
- Barlow B.K., Richfield E.K., Cory‐Slechta D.A. & Thiruchelvam M. (2003) A fetal risk factor for Parkinson's disease. Dev. Neurosci. 26, 11–23. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

