Cofactor-Free, Direct Photoactivation of Enoate Reductases for the Asymmetric Reduction of C=C Bonds
- PMID: 28544039
- PMCID: PMC5519925
- DOI: 10.1002/anie.201702461
Cofactor-Free, Direct Photoactivation of Enoate Reductases for the Asymmetric Reduction of C=C Bonds
Abstract
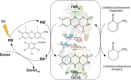
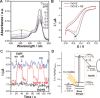
Enoate reductases from the family of old yellow enzymes (OYEs) can catalyze stereoselective trans-hydrogenation of activated C=C bonds. Their application is limited by the necessity for a continuous supply of redox equivalents such as nicotinamide cofactors [NAD(P)H]. Visible light-driven activation of OYEs through NAD(P)H-free, direct transfer of photoexcited electrons from xanthene dyes to the prosthetic flavin moiety is reported. Spectroscopic and electrochemical analyses verified spontaneous association of rose bengal and its derivatives with OYEs. Illumination of a white light-emitting-diode triggered photoreduction of OYEs by xanthene dyes, which facilitated the enantioselective reduction of C=C bonds in the absence of NADH. The photoenzymatic conversion of 2-methylcyclohexenone resulted in enantiopure (ee>99 %) (R)-2-methylcyclohexanone with conversion yields as high as 80-90 %. The turnover frequency was significantly affected by the substitution of halogen atoms in xanthene dyes.
Keywords: asymmetric reduction; enoate reductases; green chemistry; photocatalysis; redox enzymes.
© 2017 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

