Ultra-high-field magnetic resonance spectroscopy in non-alcoholic fatty liver disease: Novel mechanistic and diagnostic insights of energy metabolism in non-alcoholic steatohepatitis and advanced fibrosis
- PMID: 28544208
- PMCID: PMC5638103
- DOI: 10.1111/liv.13451
Ultra-high-field magnetic resonance spectroscopy in non-alcoholic fatty liver disease: Novel mechanistic and diagnostic insights of energy metabolism in non-alcoholic steatohepatitis and advanced fibrosis
Abstract
Background & aims: With the rising prevalence of non-alcoholic fatty liver disease (NAFLD) and steatohepatitis (NASH) non-invasive tools obtaining pathomechanistic insights to improve risk stratification are urgently needed. We therefore explored high- and ultra-high-field magnetic resonance spectroscopy (MRS) to obtain novel mechanistic and diagnostic insights into alterations of hepatic lipid, cell membrane and energy metabolism across the spectrum of NAFLD.
Methods: MRS and liver biopsy were performed in 30 NAFLD patients with NAFL (n=8) or NASH (n=22). Hepatic lipid content and composition were measured using 3-Tesla proton (1 H)-MRS. 7-Tesla phosphorus (31 P)-MRS was applied to determine phosphomonoester (PME) including phosphoethanolamine (PE), phosphodiester (PDE) including glycerophosphocholine (GPC), phosphocreatine (PCr), nicotinamide adenine dinucleotide phosphate (NADPH), inorganic phosphate (Pi), γ-ATP and total phosphorus (TP). Saturation transfer technique was used to quantify hepatic ATP flux.
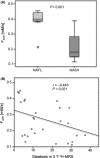
Results: Hepatic steatosis in 1 H-MRS highly correlated with histology (P<.001) showing higher values in NASH than NAFL (P<.001) without differences in saturated or unsaturated fatty acid indices. PE/TP ratio increased with advanced fibrosis (F3/4) (P=.002) whereas GPC/PME+PDE decreased (P=.05) compared to no/mild fibrosis (F0-2). γ-ATP/TP was lower in advanced fibrosis (P=.049), while PCr/TP increased (P=.01). NADPH/TP increased with higher grades of ballooning (P=.02). Pi-to-ATP exchange rate constant (P=.003) and ATP flux (P=.001) were lower in NASH than NAFL.
Conclusions: Ultra-high-field MRS, especially saturation transfer technique uncovers changes in energy metabolism including dynamic ATP flux in inflammation and fibrosis in NASH. Non-invasive profiling by MRS appears feasible and may assist further mechanistic and therapeutic studies in NAFLD/NASH.
Keywords: saturation transfer; adenosine triphosphate flux; fatty liver; lipotoxicity; mitochondrial function.
© 2017 The Authors Liver International Published by John Wiley & Sons Ltd.
Figures





References
-
- Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle‐aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124‐131. - PubMed
-
- Thomsen C, Becker U, Winkler K, Christoffersen P, Jensen M, Henriksen O. Quantification of liver fat using magnetic resonance spectroscopy. Magn Reson Imaging. 1994;12:487‐495. - PubMed
-
- Ruiz‐Cabello J, Cohen JS. Phospholipid metabolites as indicators of cancer cell function. NMR Biomed. 1992;5:226‐233. - PubMed
-
- Krssak M, Hofer H, Wrba F, et al. Non‐invasive assessment of hepatic fat accumulation in chronic hepatitis C by 1H magnetic resonance spectroscopy. Eur J Radiol. 2010;74:e60‐e66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

