Fibroblast growth factor 2 protects against renal ischaemia/reperfusion injury by attenuating mitochondrial damage and proinflammatory signalling
- PMID: 28544332
- PMCID: PMC5661260
- DOI: 10.1111/jcmm.13203
Fibroblast growth factor 2 protects against renal ischaemia/reperfusion injury by attenuating mitochondrial damage and proinflammatory signalling
Abstract
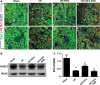
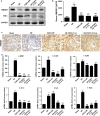
Ischaemia-reperfusion injury (I/RI) is a common cause of acute kidney injury (AKI). The molecular basis underlying I/RI-induced renal pathogenesis and measures to prevent or reverse this pathologic process remains to be resolved. Basic fibroblast growth factor (FGF2) is reported to have protective roles of myocardial infarction as well as in several other I/R related disorders. Herein we present evidence that FGF2 exhibits robust protective effect against renal histological and functional damages in a rat I/RI model. FGF2 treatment greatly alleviated I/R-induced acute renal dysfunction and largely blunted I/R-induced elevation in serum creatinine and blood urea nitrogen, and also the number of TUNEL-positive tubular cells in the kidney. Mechanistically, FGF2 substantially ameliorated renal I/RI by mitigating several mitochondria damaging parameters including pro-apoptotic alteration of Bcl2/Bax expression, caspase-3 activation, loss of mitochondrial membrane potential and KATP channel integrity. Of note, the protective effect of FGF2 was significantly compromised by the KATP channel blocker 5-HD. Interestingly, I/RI alone resulted in mild activation of FGFR, whereas FGF2 treatment led to more robust receptor activation. More significantly, post-I/RI administration of FGF2 also exhibited robust protection against I/RI by reducing cell apoptosis, inhibiting the release of damage-associated molecular pattern molecule HMBG1 and activation of its downstream inflammatory cytokines such as IL-1α, IL-6 and TNF α. Taken together, our data suggest that FGF2 offers effective protection against I/RI and improves animal survival by attenuating mitochondrial damage and HMGB1-mediated inflammatory response. Therefore, FGF2 has the potential to be used for the prevention and treatment of I/RI-induced AKI.
Keywords: High-mobility group box 1; acute kidney injury; fibroblast growth factor 2; inflammatory cytokine; ischaemia-reperfusion; mitochondrial dysfunction.
© 2017 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures


References
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

