Epithelial-to-mesenchymal transition transcription factors in cancer-associated fibroblasts
- PMID: 28544627
- PMCID: PMC5496490
- DOI: 10.1002/1878-0261.12080
Epithelial-to-mesenchymal transition transcription factors in cancer-associated fibroblasts
Abstract
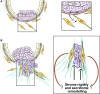
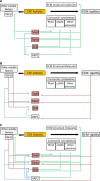
Beyond inducing epithelial-to-mesenchymal transcription (EMT), transcriptional factors of the Snail, ZEB and Twist families (EMT-TFs) control global plasticity programmes affecting cell stemness and fate. Literature addressing the reactivation of these factors in adult tumour cells is very extensive, as they enable cancer cell plasticity and fuel both tumour initiation and metastatic spread. Incipient data reveal that EMT-TFs are also expressed in fibroblasts, providing these with additional properties. Here, I will review recent reports on the expression of EMT-TFs in cancer-associated fibroblasts (CAFs). The new model suggests that EMT-TFs can be envisioned as essential metastasis and chemoresistance-promoting molecules, thereby enabling coordinated plasticity programmes in parenchyma and stroma-tumour compartments.
Keywords: ZEB; Snail; Twist; cancer-associated fibroblasts; epithelial-to-mesenchymal transition transcription factors; tumour-stroma crosstalk.
© 2017 The Authors. Published by FEBS Press and John Wiley & Sons Ltd.
Figures
References
-
- Alba‐Castellón L, Olivera‐Salguero R, Mestre‐Farrera A, Peña R, Herrera M, Bonilla F, Casal JI, Baulida J, Peña C and García de Herreros A (2016) Snail1‐dependent activation of cancer‐associated fibroblast controls epithelial tumor cell invasion and metastasis. Cancer Res 76, 6205–6217. - PubMed
-
- Ansieau S, Bastid J, Doreau A, Morel A‐P, Bouchet BP, Thomas C, Fauvet F, Puisieux I, Doglioni C, Piccinin S et al (2008) Induction of EMT by twist proteins as a collateral effect of tumor‐promoting inactivation of premature senescence. Cancer Cell 14, 79–89. - PubMed
-
- Ansieau S, Morel A‐P, Hinkal G, Bastid J and Puisieux A (2010) TWISTing an embryonic transcription factor into an oncoprotein. Oncogene 29, 3173–3184. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

