Diverse application of MRI for mouse phenotyping
- PMID: 28544650
- PMCID: PMC5738275
- DOI: 10.1002/bdr2.1051
Diverse application of MRI for mouse phenotyping
Abstract
Small animal models, particularly mouse models, of human diseases are becoming an indispensable tool for biomedical research. Studies in animal models have provided important insights into the etiology of diseases and accelerated the development of therapeutic strategies. Detailed phenotypic characterization is essential, both for the development of such animal models and mechanistic studies into disease pathogenesis and testing the efficacy of experimental therapeutics. MRI is a versatile and noninvasive imaging modality with excellent penetration depth, tissue coverage, and soft tissue contrast. MRI, being a multi-modal imaging modality, together with proven imaging protocols and availability of good contrast agents, is ideally suited for phenotyping mutant mouse models. Here we describe the applications of MRI for phenotyping structural birth defects involving the brain, heart, and kidney in mice. The versatility of MRI and its ease of use are well suited to meet the rapidly increasing demands for mouse phenotyping in the coming age of functional genomics. Birth Defects Research 109:758-770, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: MRI; brain; congenital malformation; diffusion tensor imaging; heart; kidney; magnetic resonance Imaging; mouse; mutant; tagging.
© 2017 Wiley Periodicals, Inc.
Figures
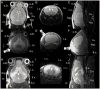
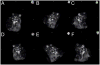
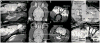
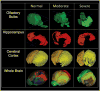




Similar articles
-
Cardiac MRI Assessment of Mouse Myocardial Infarction and Regeneration.Methods Mol Biol. 2021;2158:81-106. doi: 10.1007/978-1-0716-0668-1_8. Methods Mol Biol. 2021. PMID: 32857368
-
Phenotyping cardiac and structural birth defects in fetal and newborn mice.Birth Defects Res. 2017 Jun 1;109(10):778-790. doi: 10.1002/bdr2.1048. Epub 2017 May 22. Birth Defects Res. 2017. PMID: 28544620 Free PMC article. Review.
-
High-throughput magnetic resonance imaging in mice for phenotyping and therapeutic evaluation.Curr Opin Chem Biol. 2005 Aug;9(4):413-20. doi: 10.1016/j.cbpa.2005.06.004. Curr Opin Chem Biol. 2005. PMID: 16002325 Review.
-
Mouse phenotyping with MRI.Methods Mol Biol. 2011;771:595-631. doi: 10.1007/978-1-61779-219-9_31. Methods Mol Biol. 2011. PMID: 21874500
-
Imaging techniques for visualizing and phenotyping congenital heart defects in murine models.Birth Defects Res C Embryo Today. 2013 Jun;99(2):93-105. doi: 10.1002/bdrc.21037. Birth Defects Res C Embryo Today. 2013. PMID: 23897594 Free PMC article. Review.
Cited by
-
Paired immunoglobulin-like receptor B is an entry receptor for mammalian orthoreovirus.Nat Commun. 2023 May 5;14(1):2615. doi: 10.1038/s41467-023-38327-6. Nat Commun. 2023. PMID: 37147336 Free PMC article.
-
Comprehensive Volumetric Analysis of Mecp2-Null Mouse Model for Rett Syndrome by T2-Weighted 3D Magnetic Resonance Imaging.Front Neurosci. 2022 May 10;16:885335. doi: 10.3389/fnins.2022.885335. eCollection 2022. Front Neurosci. 2022. PMID: 35620663 Free PMC article.
-
Non-invasive Free-breathing Gating-free Extracellular Cellular Volume Quantification for Repetitive Myocardial Fibrosis Evaluation in Rodents.bioRxiv [Preprint]. 2025 Jul 17:2025.07.11.664411. doi: 10.1101/2025.07.11.664411. bioRxiv. 2025. PMID: 40791328 Free PMC article. Preprint.
-
Monocyte depletion attenuates the development of posttraumatic hydrocephalus and preserves white matter integrity after traumatic brain injury.PLoS One. 2018 Nov 1;13(11):e0202722. doi: 10.1371/journal.pone.0202722. eCollection 2018. PLoS One. 2018. PMID: 30383765 Free PMC article.
-
Genome Editing and Myocardial Development.Adv Exp Med Biol. 2023;1396:53-73. doi: 10.1007/978-981-19-5642-3_4. Adv Exp Med Biol. 2023. PMID: 36454459 Review.
References
-
- Ackerman JJH, Neil JJ. Biophysics of Diffusion in Cells. In: Jones DK, editor. Diffusion MRI: Theory, Methods and Applications. Oxford U. Press; Oxford: 2010. pp. 110–124.
-
- Ahrens ET, Laidlaw DH, Readhead C, Brosnan CF, Fraser SE, Jacobs RE. MR microscopy of transgenic mice that spontaneously acquire experimental allergic encephalomyelitis. Magnetic resonance in medicine. 1998;40:119–132. - PubMed
-
- Alexander DC, Barker GJ. Optimal imaging parameters for fiber-orientation estimation in diffusion MRI. NeuroImage. 2005;27:357–367. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

