Cyclotides: Overview and Biotechnological Applications
- PMID: 28544675
- PMCID: PMC5812342
- DOI: 10.1002/cbic.201700153
Cyclotides: Overview and Biotechnological Applications
Abstract
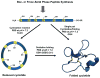
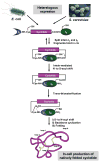
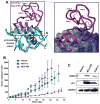
Cyclotides are globular microproteins with a unique head-to-tail cyclized backbone, stabilized by three disulfide bonds forming a cystine knot. This unique circular backbone topology and knotted arrangement of three disulfide bonds makes them exceptionally stable to chemical, thermal, and biological degradation compared to other peptides of similar size. In addition, cyclotides have been shown to be highly tolerant to sequence variability, aside from the conserved residues forming the cystine knot. Cyclotides can also cross cellular membranes and are able to modulate intracellular protein-protein interactions, both in vitro and in vivo. All of these features make cyclotides highly promising as leads or frameworks for the design of peptide-based diagnostic and therapeutic tools. This article provides an overview on cyclotides and their applications as molecular imaging agents and peptide-based therapeutics.
Keywords: cyclic peptides; cyclotides; cystine knots; drug design; imaging agents.
© 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
Similar articles
-
Cyclotides, a versatile ultrastable micro-protein scaffold for biotechnological applications.Bioorg Med Chem Lett. 2017 Dec 1;27(23):5089-5099. doi: 10.1016/j.bmcl.2017.10.051. Epub 2017 Oct 21. Bioorg Med Chem Lett. 2017. PMID: 29110985 Free PMC article. Review.
-
Using backbone-cyclized Cys-rich polypeptides as molecular scaffolds to target protein-protein interactions.Biochem J. 2019 Jan 11;476(1):67-83. doi: 10.1042/BCJ20180792. Biochem J. 2019. PMID: 30635453 Free PMC article. Review.
-
The Potential of the Cyclotide Scaffold for Drug Development.Biomedicines. 2019 Apr 19;7(2):31. doi: 10.3390/biomedicines7020031. Biomedicines. 2019. PMID: 31010257 Free PMC article. Review.
-
Cyclotides as Tools in Chemical Biology.Acc Chem Res. 2017 Jul 18;50(7):1557-1565. doi: 10.1021/acs.accounts.7b00157. Epub 2017 Jun 23. Acc Chem Res. 2017. PMID: 28644007
-
Biological activities of natural and engineered cyclotides, a novel molecular scaffold for peptide-based therapeutics.Curr Mol Pharmacol. 2010 Nov;3(3):153-63. doi: 10.2174/1874467211003030153. Curr Mol Pharmacol. 2010. PMID: 20858197 Free PMC article. Review.
Cited by
-
Bacterial fermentation and isotope labelling optimized for amyloidogenic proteins.Microb Biotechnol. 2021 May;14(3):1107-1119. doi: 10.1111/1751-7915.13778. Epub 2021 Mar 19. Microb Biotechnol. 2021. PMID: 33739615 Free PMC article.
-
Isolation and characterization of cyclotides from the leaves of Viola odorata L. using peptidomic and bioinformatic approach.3 Biotech. 2021 May;11(5):211. doi: 10.1007/s13205-021-02763-2. Epub 2021 Apr 11. 3 Biotech. 2021. PMID: 33927999 Free PMC article.
-
Potent, Selective, and Cell-Penetrating Inhibitors of Kallikrein-Related Peptidase 4 Based on the Cyclic Peptide MCoTI-II.ACS Med Chem Lett. 2018 Nov 21;9(12):1258-1262. doi: 10.1021/acsmedchemlett.8b00422. eCollection 2018 Dec 13. ACS Med Chem Lett. 2018. PMID: 30613336 Free PMC article.
-
Recombinant Expression of Cyclotides Using Expressed Protein Ligation.Methods Mol Biol. 2020;2133:327-341. doi: 10.1007/978-1-0716-0434-2_16. Methods Mol Biol. 2020. PMID: 32144675 Free PMC article.
-
Comprehensive Mapping of Cyclotides from Viola philippica by Using Mass Spectrometry-Based Strategy.Molecules. 2024 Sep 13;29(18):4344. doi: 10.3390/molecules29184344. Molecules. 2024. PMID: 39339338 Free PMC article.
References
-
- Pluckthun A. Annu Rev Pharmacol Toxicol. 2015;55:489–511. - PubMed
- Liu H, Saxena A, Sidhu SS, Wu D. Front Immunol. 2017;8:38. - PMC - PubMed
- Krah S, Schroter C, Zielonka S, Empting M, Valldorf B, Kolmar H. Immunopharmacol Immunotoxicol. 2016;38:21–28. - PubMed
- Kintzing JR, Filsinger Interrante MV, Cochran JR. Trends Pharmacol Sci. 2016;37:993–1008. - PMC - PubMed
-
- McGregor DP. Curr Opin Pharmacol. 2008;8:616–619. - PubMed
-
- Kintzing JR, Cochran JR. Curr Opin Chem Biol. 2016;34:143–150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

