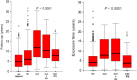
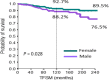
Role of treatment on the development of secondary malignancies in patients with essential thrombocythemia
- PMID: 28544749
- PMCID: PMC5463060
- DOI: 10.1002/cam4.1081
Role of treatment on the development of secondary malignancies in patients with essential thrombocythemia
Abstract
Aim of this study is to explore the role of different treatments on the development of secondary malignancies (SMs) in a large cohort of essential thrombocythemia (ET) patients. We report the experience of a regional cooperative group in a real-life cohort of 1026 patients with ET. We divided our population into five different groups: group 0, no treatment; group 1, hydroxyurea (HU); group 2, alkylating agents (ALK); group 3, ALK + HU sequentially or in combination; and group 4, anagrelide (ANA) and/or α-interferon (IFN) only. Patients from groups 1, 2, and 3 could also have been treated either with ANA and/or IFN in their medical history, considering these drugs not to have an additional cytotoxic potential. In all, 63 of the 1026 patients (6%) developed 64 SM during the follow-up, after a median time of 50 months (range: 2-158) from diagnosis. In univariate analysis, a statistically significant difference was found only for gender (P = 0.035) and age (P = 0.0001). In multivariate analysis, a statistically significant difference was maintained for both gender and age (gender HR1.7 [CI 95% 1.037-2.818] P = 0.035; age HR 4.190 [CI 95% 2.308-7.607] P = 0.0001). The impact of different treatments on SMs development was not statistically significant. In our series of 1026 ET patients, diagnosed and followed during a 30-year period, the different therapies administered, comprising HU and ALK, do not appear to have impacted on the development of SM. A similar rate of SMs was observed also in untreated patients. The only two variables which showed a statistical significance were male gender and age >60 years.
Keywords: Alkylating agents; alpha-interferon; anagrelide; essential thrombocythemia; hydroxyurea; secondary malignancy.
© 2017 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Figures
References
-
- Fenaux, P. , Simon M., Caulier M. T., Lai J. L., Goudemand J., and Bauters F.. 1990. Clinical course of essential thrombocythemia in 147 cases. Cancer. 66:549–556. - PubMed
-
- Cortelazzo, S. , Viero P., Finazzi G., D'Emilio A., Rodeghiero F., and Barbui T.. 1990. Incidence and risk factors for thrombotic complications in a historical cohort of 100 patients with essential thrombocythemia. J Clin Oncol. 8:556–562. - PubMed
-
- Passamonti, F. , Rumi E., Arcaini L., Boveri E., Elena C., Pietra D., et al. 2008. Prognostic factors for thrombosis, myelofibrosis, and leukemia in essential thrombocythemia: a study of 605 patients. Haematologica. 93:1645–1651. - PubMed
-
- Passamonti, F. , Rumi E., Pungolino E., Malabarba L., Bertazzoni P., Valentini M., et al. 2004. Life expectancy and prognostic factors for survival in patients with polycythemia vera and essential thrombocythemia. Am J Med. 117:755–761. - PubMed
-
- Tefferi, A. , and Murphy S.. 2001. Current opinion in essential thrombocythemia: pathogenesis, diagnosis, and management. Blood Rev. 15:121–131. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources