Neural oscillations demonstrate that general anesthesia and sedative states are neurophysiologically distinct from sleep
- PMID: 28544930
- PMCID: PMC5520989
- DOI: 10.1016/j.conb.2017.04.011
Neural oscillations demonstrate that general anesthesia and sedative states are neurophysiologically distinct from sleep
Abstract
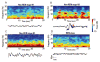
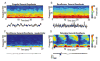
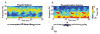
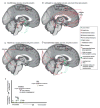
General anesthesia is a man-made neurophysiological state comprised of unconsciousness, amnesia, analgesia, and immobility along with maintenance of physiological stability. Growing evidence suggests that anesthetic-induced neural oscillations are a primary mechanism of anesthetic action. Each anesthetic drug class produces distinct oscillatory dynamics that can be related to the circuit mechanisms of drug action. Sleep is a naturally occurring state of decreased arousal that is essential for normal health. Physiological measurements (electrooculogram, electromyogram) and neural oscillatory (electroencephalogram) dynamics are used to empirically characterize sleep into rapid eye movement sleep and the three stages of non-rapid eye movement sleep. In this review, we discuss the differences between anesthesia- and sleep-induced altered states from the perspective of neural oscillations.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

