Intranasal delivery of a bivalent norovirus vaccine formulated in an in situ gelling dry powder
- PMID: 28545100
- PMCID: PMC5436670
- DOI: 10.1371/journal.pone.0177310
Intranasal delivery of a bivalent norovirus vaccine formulated in an in situ gelling dry powder
Abstract
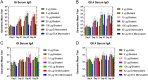
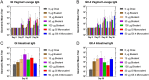
The global health community is beginning to understand the burden of norovirus-associated disease, which has a significant impact in both developed and developing countries. Norovirus virus like particle (VLP)-based vaccines are currently under development and have been shown to elicit systemic and mucosal immune responses when delivered intranasally. In the present study, we describe the use of a dry powder formulation (GelVac™) with an in situ gelling polysaccharide (GelSite™) extracted from Aloe vera for nasal delivery of a bivalent vaccine formulation containing both GI and GII.4 norovirus VLPs. Dose-ranging studies were performed to identify the optimal antigen dosages based on systemic and mucosal immune responses in guinea pigs and determine any antigenic interference. A dose-dependent increase in systemic and mucosal immunogenicity against each of the VLPs were observed as well as a boosting effect for each VLP after the second dosing. A total antigen dose of ≥50 μg of each GI and GII.4 VLPs was determined to be the maximally immunogenic dose in guinea pigs. The immunogenicity results of this bivalent formulation, taken together with previous work on monovalent GelVac™ norovirus vaccine formulation, provides a basis for future development of this norovirus VLP vaccine.
Conflict of interest statement
Figures


Similar articles
-
Preclinical dose-ranging studies of a novel dry powder norovirus vaccine formulation.Vaccine. 2016 Mar 14;34(12):1452-8. doi: 10.1016/j.vaccine.2016.01.064. Epub 2016 Feb 10. Vaccine. 2016. PMID: 26873053 Free PMC article.
-
Intranasal delivery of Norwalk virus-like particles formulated in an in situ gelling, dry powder vaccine.Vaccine. 2011 Jul 18;29(32):5221-31. doi: 10.1016/j.vaccine.2011.05.027. Epub 2011 Jun 2. Vaccine. 2011. PMID: 21640778 Free PMC article.
-
Intranasal P particle vaccine provided partial cross-variant protection against human GII.4 norovirus diarrhea in gnotobiotic pigs.J Virol. 2014 Sep 1;88(17):9728-43. doi: 10.1128/JVI.01249-14. Epub 2014 Jun 11. J Virol. 2014. PMID: 24920797 Free PMC article.
-
Progress on norovirus vaccine research: public health considerations and future directions.Expert Rev Vaccines. 2018 Sep;17(9):773-784. doi: 10.1080/14760584.2018.1510327. Epub 2018 Aug 27. Expert Rev Vaccines. 2018. PMID: 30092671 Free PMC article. Review.
-
Norovirus Vaccine: Priorities for Future Research and Development.Front Immunol. 2020 Jul 7;11:1383. doi: 10.3389/fimmu.2020.01383. eCollection 2020. Front Immunol. 2020. PMID: 32733458 Free PMC article. Review.
Cited by
-
Efficient oral vaccination by bioengineering virus-like particles with protozoan surface proteins.Nat Commun. 2019 Jan 21;10(1):361. doi: 10.1038/s41467-018-08265-9. Nat Commun. 2019. PMID: 30664644 Free PMC article.
-
Norovirus-Associated Gastroenteritis Vesikari Score and Pre-Existing Salivary IgA in Young Children from Rural South Africa.Viruses. 2023 Oct 30;15(11):2185. doi: 10.3390/v15112185. Viruses. 2023. PMID: 38005863 Free PMC article.
-
A Bibliometric Analysis of the Literature on Norovirus Disease from 1991-2021.Int J Environ Res Public Health. 2022 Feb 22;19(5):2508. doi: 10.3390/ijerph19052508. Int J Environ Res Public Health. 2022. PMID: 35270203 Free PMC article.
-
Intranasal immunization with aluminum salt-adjuvanted dry powder vaccine.J Control Release. 2018 Dec 28;292:111-118. doi: 10.1016/j.jconrel.2018.10.020. Epub 2018 Oct 16. J Control Release. 2018. PMID: 30339906 Free PMC article.
-
Parenterally Administered Norovirus GII.4 Virus-Like Particle Vaccine Formulated with Aluminum Hydroxide or Monophosphoryl Lipid A Adjuvants Induces Systemic but Not Mucosal Immune Responses in Mice.J Immunol Res. 2018 Feb 28;2018:3487095. doi: 10.1155/2018/3487095. eCollection 2018. J Immunol Res. 2018. PMID: 29682589 Free PMC article.
References
-
- Caul EO. Small round structured viruses: airborne transmission and hospital control. Lancet. 1994;343(8908):1240–2. . - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

