Intranasal delivery of a bivalent norovirus vaccine formulated in an in situ gelling dry powder
- PMID: 28545100
- PMCID: PMC5436670
- DOI: 10.1371/journal.pone.0177310
Intranasal delivery of a bivalent norovirus vaccine formulated in an in situ gelling dry powder
Abstract
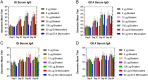
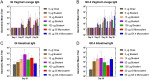
The global health community is beginning to understand the burden of norovirus-associated disease, which has a significant impact in both developed and developing countries. Norovirus virus like particle (VLP)-based vaccines are currently under development and have been shown to elicit systemic and mucosal immune responses when delivered intranasally. In the present study, we describe the use of a dry powder formulation (GelVac™) with an in situ gelling polysaccharide (GelSite™) extracted from Aloe vera for nasal delivery of a bivalent vaccine formulation containing both GI and GII.4 norovirus VLPs. Dose-ranging studies were performed to identify the optimal antigen dosages based on systemic and mucosal immune responses in guinea pigs and determine any antigenic interference. A dose-dependent increase in systemic and mucosal immunogenicity against each of the VLPs were observed as well as a boosting effect for each VLP after the second dosing. A total antigen dose of ≥50 μg of each GI and GII.4 VLPs was determined to be the maximally immunogenic dose in guinea pigs. The immunogenicity results of this bivalent formulation, taken together with previous work on monovalent GelVac™ norovirus vaccine formulation, provides a basis for future development of this norovirus VLP vaccine.
Conflict of interest statement
Figures


References
-
- Caul EO. Small round structured viruses: airborne transmission and hospital control. Lancet. 1994;343(8908):1240–2. . - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

