Thermostabilization of the uronate dehydrogenase from Agrobacterium tumefaciens by semi-rational design
- PMID: 28545260
- PMCID: PMC5442039
- DOI: 10.1186/s13568-017-0405-2
Thermostabilization of the uronate dehydrogenase from Agrobacterium tumefaciens by semi-rational design
Abstract
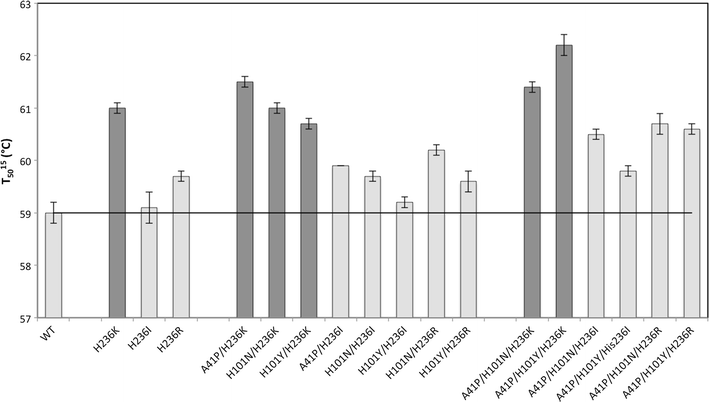
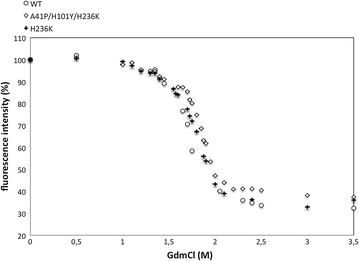
Aldaric acids represent biobased 'top value-added chemicals' that have the potential to substitute petroleum-derived chemicals. Until today they are mostly produced from corresponding aldoses using strong chemical oxidizing agents. An environmentally friendly and more selective process could be achieved by using natural resources such as seaweed or pectin as raw material. These contain large amounts of uronic acids as major constituents such as glucuronic acid and galacturonic acid which can be converted into the corresponding aldaric acids via an enzyme-based oxidation using uronate dehydrogenase (Udh). The Udh from Agrobacterium tumefaciens (UdhAt) features the highest catalytic efficiency of all characterized Udhs using glucuronic acid as substrate (829 s-1 mM-1). Unfortunately, it suffers from poor thermostability. To overcome this limitation, we created more thermostable variants using semi-rational design. The amino acids for substitution were chosen according to the B factor in combination with four additional knowledge-based criteria. The triple variant A41P/H101Y/H236K showed higher kinetic and thermodynamic stability with a T 5015 value of 62.2 °C (3.2 °C improvement) and a ∆∆GU of 2.3 kJ/mol compared to wild type. Interestingly, it was only obtained when including a neutral mutation in the combination.
Keywords: Agrobacterium tumefaciens; B factor; Glucuronic acid; Neutral drift; Thermostability; Uronate dehydrogenase.
Figures


Similar articles
-
Characterization of uronate dehydrogenases catalysing the initial step in an oxidative pathway.Microb Biotechnol. 2015 Jul;8(4):633-43. doi: 10.1111/1751-7915.12265. Epub 2015 Apr 17. Microb Biotechnol. 2015. PMID: 25884328 Free PMC article.
-
Substrate-imprinted docking of Agrobacterium tumefaciens uronate dehydrogenase for increased substrate selectivity.Int J Biol Macromol. 2019 Nov 1;140:1214-1225. doi: 10.1016/j.ijbiomac.2019.08.194. Epub 2019 Aug 28. Int J Biol Macromol. 2019. PMID: 31472210
-
Characterization of a uronate dehydrogenase from Thermobispora bispora for production of glucaric acid from hemicellulose substrate.World J Microbiol Biotechnol. 2018 Jun 23;34(7):102. doi: 10.1007/s11274-018-2486-8. World J Microbiol Biotechnol. 2018. PMID: 29936649
-
Production and applications of carbohydrate-derived sugar acids as generic biobased chemicals.Crit Rev Biotechnol. 2016 Oct;36(5):904-16. doi: 10.3109/07388551.2015.1060189. Epub 2015 Jul 15. Crit Rev Biotechnol. 2016. PMID: 26177333 Review.
-
[Production of mucic acid from pectin-derived D-galacturonic acid: a review].Sheng Wu Gong Cheng Xue Bao. 2022 Feb 25;38(2):666-677. doi: 10.13345/j.cjb.210268. Sheng Wu Gong Cheng Xue Bao. 2022. PMID: 35234389 Review. Chinese.
Cited by
-
Chromohalobacter salixigens Uronate Dehydrogenase: Directed Evolution for Improved Thermal Stability and Mutant CsUDH-inc X-ray Crystal Structure.Process Biochem. 2022 Mar;114:185-192. doi: 10.1016/j.procbio.2020.02.013. Epub 2020 Feb 14. Process Biochem. 2022. PMID: 35462854 Free PMC article.
-
Enhancing the Thermostability of Rhizomucor miehei Lipase with a Limited Screening Library by Rational-Design Point Mutations and Disulfide Bonds.Appl Environ Microbiol. 2018 Jan 2;84(2):e02129-17. doi: 10.1128/AEM.02129-17. Print 2018 Jan 15. Appl Environ Microbiol. 2018. PMID: 29101200 Free PMC article.
-
Thermostability engineering of industrial enzymes through structure modification.Appl Microbiol Biotechnol. 2022 Aug;106(13-16):4845-4866. doi: 10.1007/s00253-022-12067-x. Epub 2022 Jul 9. Appl Microbiol Biotechnol. 2022. PMID: 35804158 Review.
References
-
- Andberg M, Maaheimo H, Boer H, Penttilä M, Koivula A, Richard P. Characterization of a novel Agrobacterium tumefaciens galactarolactone cycloisomerase enzyme for direct conversion of d-galactarolactone to 3-deoxy-2-keto-l-threo-hexarate. J Biol Chem. 2012;287(21):17662–17671. doi: 10.1074/jbc.M111.335240. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

