Optimized methods for rapidly dissecting spinal cords and harvesting spinal motor neurons with high survival and purity from rats at different embryonic stages
- PMID: 28545340
- PMCID: PMC6055952
- DOI: 10.1080/10790268.2017.1329075
Optimized methods for rapidly dissecting spinal cords and harvesting spinal motor neurons with high survival and purity from rats at different embryonic stages
Abstract
Study design: Experimental study, protocol optimization.
Objectives: To investigate and compare the isolation of spinal motor neurons from embryonic rats at different embryonic stages, and develop optimized methods for rapidly dissecting spinal cords and harvesting spinal motor neurons with high survival and purity.
Setting: Guangdong Provincial Academy of Chinese Medical Sciences, Guangzhou, China.
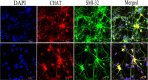
Methods: Embryonic rats at different embryonic stages (12-18 days) were used to isolate spinal motor neurons. Their shape and corresponding dissection procedures, time needed and skills were compared. After dissecting and dissociating spinal cords, cells were randomly divided into immunopanning group and control group, in which antibodies to p75NTR were used or not. After plating cells, different recipe were added at different stages in serum-free culture media. Morphological features of cells were observed during development. Immunoflurorescence assay was performed to indentify motor neurons and the proportion of motor neurons in both control and immunopanning group were evaluated and compared.
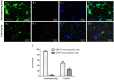
Results: We summarized the operation essentials for rapid isolation of spinal cords, as well as compared anatomical features and dissection procedures of embryos at different embryonic stages, which help us to better evaluate the developmental profile and isolate cells by adopting corresponding skills. Through the fast isolation procedure and optimized culture media, cells grow in good viability. Moreover, compared with control group, the purity of spinal motor neurons in the immunopanning group was significantly increased, reaching a proportion of over 95%.
Keywords: Embryos; High purity; Immunopanning; Spinal cords; Spinal motor neurons; Survival of motor neuron.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
