A plea to provide best evidence in trials under sample-size restrictions: the example of pioglitazone to resolve leukoplakia and erythroplakia in Fanconi anemia patients
- PMID: 28545482
- PMCID: PMC5445360
- DOI: 10.1186/s13023-017-0655-8
A plea to provide best evidence in trials under sample-size restrictions: the example of pioglitazone to resolve leukoplakia and erythroplakia in Fanconi anemia patients
Abstract
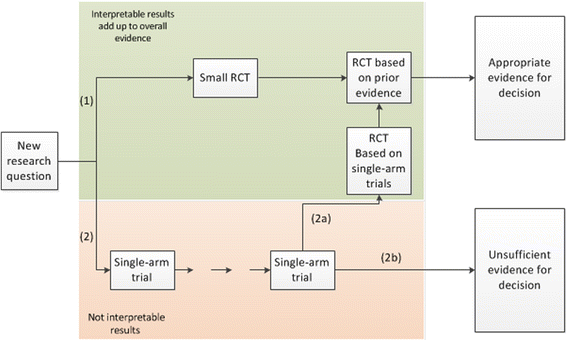
In planning a clinical trial for demonstrating the efficacy of pioglitazone to resolve leukoplakia and erythroplakia in Fanconi anemia patients we had to discuss the need for a randomized controlled trial particularly under sample-size restrictions as very promising results were available from a single-arm clinical trial. Unfortunately, at a later stage, we had to suffer from the fact that single-arm clinical trials may sometimes mislead. When revisiting our planning at a later stage of a grant application, results of a randomized controlled trial had become available which were less impressive, but may still be of clinical interest. However, these results were perceived as disappointing in the light of previously raised hopes based on the results of the single-arm trial. We highlight some major problems when research is based on single-arm trials compared to randomized controlled trials. After debunking common arguments for the conduct of single-arm trials in rare disease we conclude that particularly in rare disease research should be based on randomized building blocks simply because more robust evidence is generated. The plea for single-arm trials should be substituted by a plea for cooperation of all stakeholders to provide best evidence for decision making under sample-size restrictions.
Keywords: Evidentiary standards; Fanconi anemia; Historical control; RCT; Randomization; Rare disease; Single-arm trial; Study planning.
References
-
- Bell SA, Tudur Smith C. A comparison of interventional clinical trials in rare versus non-rare diseases: an analysis of ClinicalTrials.gov. Orphanet J Rare Dis. 2014;9. - PMC - PubMed
-
- EMA . Guideline on clinical trials in small populations. 2006. pp. 1–10.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources