Open-label versus double-blind placebo treatment in irritable bowel syndrome: study protocol for a randomized controlled trial
- PMID: 28545508
- PMCID: PMC5445390
- DOI: 10.1186/s13063-017-1964-x
Open-label versus double-blind placebo treatment in irritable bowel syndrome: study protocol for a randomized controlled trial
Abstract
Background: Placebo medications, by definition, are composed of inactive ingredients that have no physiological effect on symptoms. Nonetheless, administration of placebo in randomized controlled trials (RCTs) and in clinical settings has been demonstrated to have significant impact on many physical and psychological complaints. Until recently, conventional wisdom has suggested that patients must believe that placebo pills actually contain (or, at least, might possibly contain) active medication in order to elicit a response to placebo. However, several recent RCTs, including patients with irritable bowel syndrome (IBS), chronic low back pain, and episodic migraine, have demonstrated that individuals receiving open-label placebo (OLP) can still experience symptomatic improvement and benefit from honestly described placebo treatment.
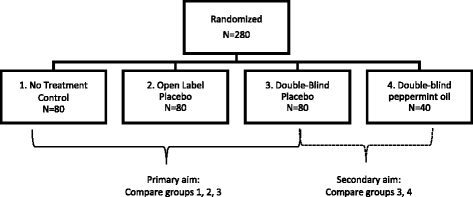
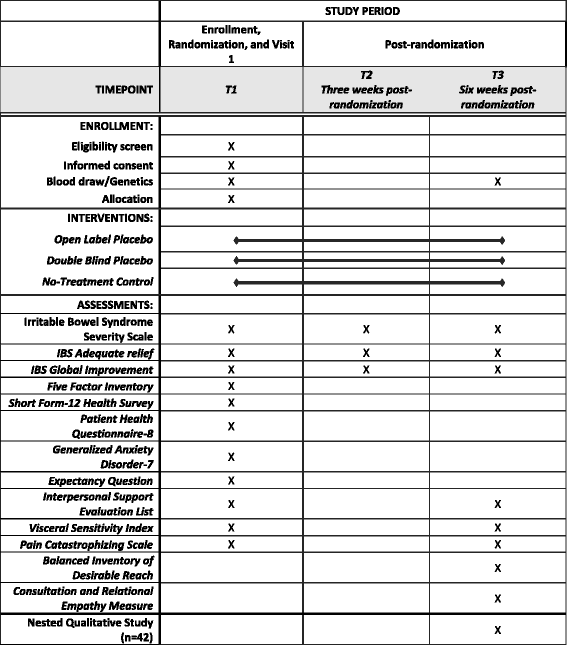
Methods and design: This paper describes an innovative multidisciplinary trial design (n = 280) that attempts to replicate and expand upon an earlier IBS OLP study. The current study will compare OLP to double-blind placebo (DBP) administration which is made possible by including a nested, double-blind RCT comparing DBP and peppermint oil. The study also examines possible genetic and psychological predictors of OLP and seeks to better understand participants' experiences with OLP and DBP through a series of extensive interviews with a randomly selected subgroup.
Discussion: OLP treatment is a novel strategy for ethically harnessing placebo effects. It has potential to re-frame theories of placebo and to influence how physicians can optimize watch-and-wait strategies for common, subjective symptoms. The current study aims to dramatically expand what we know about OLP by comparing, for the first time, OLP and DBP administration. Adopting a unique, multidisciplinary approach, the study also explores genetic, psychological and experiential dimensions of OLP. The paper ends with an extensive discussion of the "culture" of the trial as well as potential mechanisms of OLP and ethical implications.
Trial registration: ClinicalTrials.gov, identifier: NCT02802241 . Registered on 14 June 2016.
Keywords: Irritable bowel syndrome; Multi-disciplinary models; Open-label placebo; Peppermint oil; Placebo effects; Research methods.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical