hrHPV E5 oncoprotein: immune evasion and related immunotherapies
- PMID: 28545552
- PMCID: PMC5445378
- DOI: 10.1186/s13046-017-0541-1
hrHPV E5 oncoprotein: immune evasion and related immunotherapies
Abstract
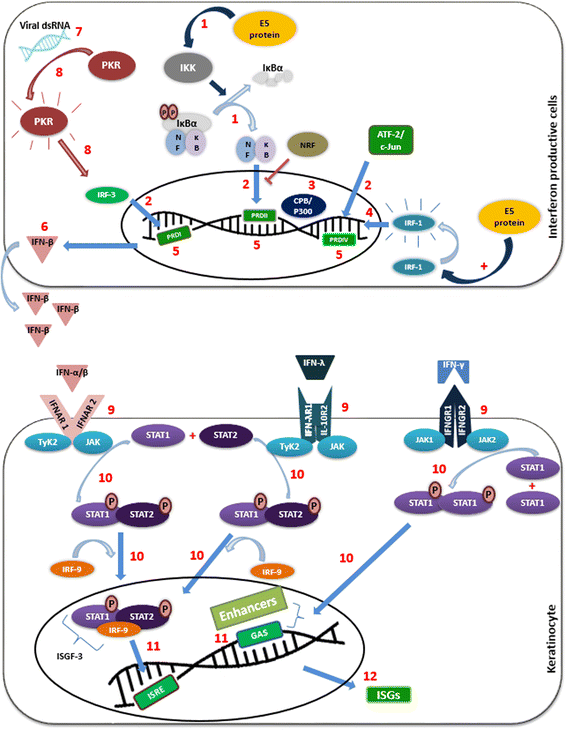
The immune response is a key factor in the fight against HPV infection and related cancers, and thus, HPV is able to promote immune evasion through the expression of oncogenes. In particular, the E5 oncogene is responsible for modulation of several immune mechanisms, including antigen presentation and inflammatory pathways. Moreover, E5 was suggested as a promising therapeutic target, since there is still no effective medical therapy for the treatment of HPV-related pre-neoplasia and cancer. Indeed, several studies have shown good prospective for E5 immunotherapy, suggesting that it could be applied for the treatment of pre-cancerous lesions. Thus, insofar as the majority of cervical, oropharyngeal and anal cancers are caused by high-risk HPV (hrHPV), mainly by HPV16, the aim of this review is to discuss the immune pathways interfered by E5 oncoprotein of hrHPV highlighting the various aspects of the potential immunotherapeutic approaches.
Keywords: E5 oncoprotein; HPV immune evasion; HPV-related cancer; Human Papillomavirus; Immune response modulation; Immunotherapy.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

