Evaluation of a laboratory quality assurance pilot programme for malaria diagnostics in low-transmission areas of Kenya, 2013
- PMID: 28545579
- PMCID: PMC5445328
- DOI: 10.1186/s12936-017-1856-2
Evaluation of a laboratory quality assurance pilot programme for malaria diagnostics in low-transmission areas of Kenya, 2013
Abstract
Background: One objective of the Kenya National Malaria Strategy 2009-2017 is scaling access to prompt diagnosis and effective treatment. In 2013, a quality assurance (QA) pilot was implemented to improve accuracy of malaria diagnostics at selected health facilities in low-transmission counties of Kenya. Trends in malaria diagnostic and QA indicator performance during the pilot are described.
Methods: From June to December 2013, 28 QA officers provided on-the-job training and mentoring for malaria microscopy, malaria rapid diagnostic tests and laboratory QA/quality control (QC) practices over four 1-day visits at 83 health facilities. QA officers observed and recorded laboratory conditions and practices and cross-checked blood slides for malaria parasite presence, and a portion of cross-checked slides were confirmed by reference laboratories.
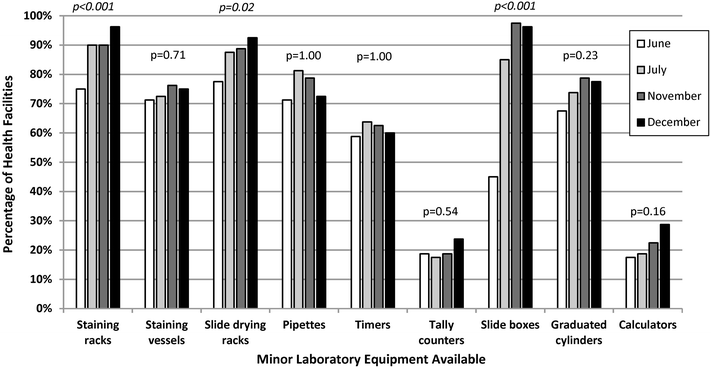
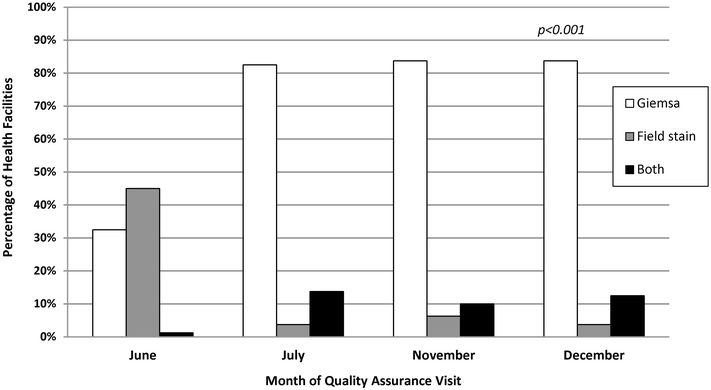
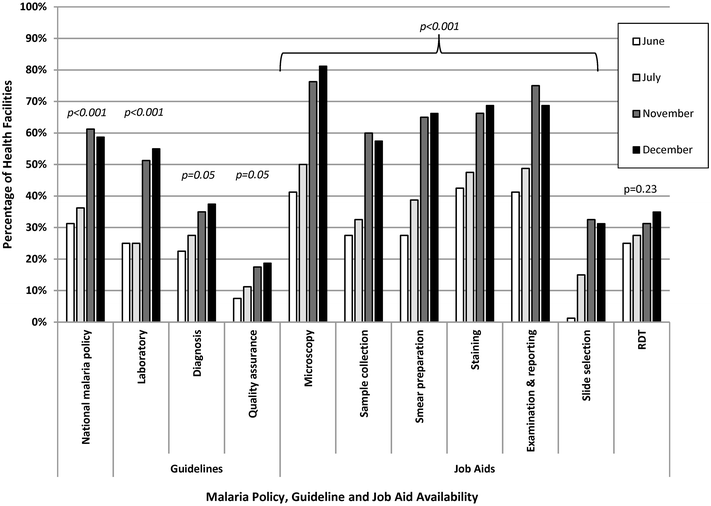
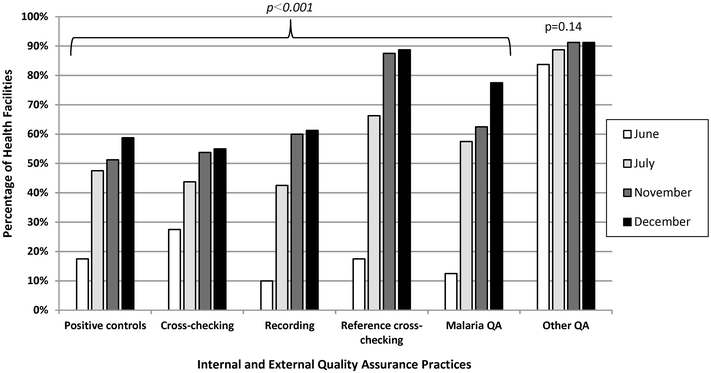
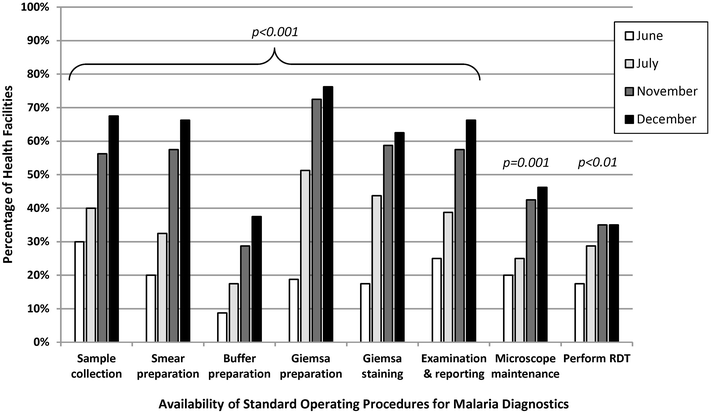
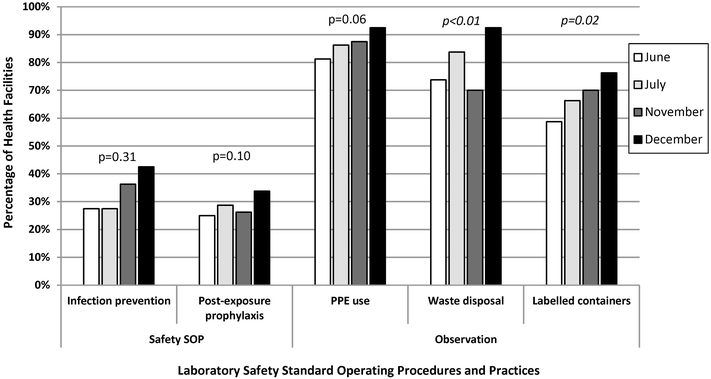
Results: Eighty (96%) facilities completed the pilot. Among 315 personnel at pilot initiation, 13% (n = 40) reported malaria diagnostics training within the previous 12 months. Slide positivity ranged from 3 to 7%. Compared to the reference laboratory, microscopy sensitivity ranged from 53 to 96% and positive predictive value from 39 to 53% for facility staff and from 60 to 96% and 52 to 80%, respectively, for QA officers. Compared to reference, specificity ranged from 88 to 98% and negative predictive value from 98 to 99% for health-facility personnel and from 93 to 99% and 99%, respectively, for QA officers. The kappa value ranged from 0.48-0.66 for facility staff and 0.57-0.84 for QA officers compared to reference. The only significant test performance improvement observed for facility staff was for specificity from 88% (95% CI 85-90%) to 98% (95% CI 97-99%). QA/QC practices, including use of positive-control slides, internal and external slide cross-checking and recording of QA/QC activities, all increased significantly across the pilot (p < 0.001). Reference material availability also increased significantly; availability of six microscopy job aids and seven microscopy standard operating procedures increased by a mean of 32 percentage points (p < 0.001) and 38 percentage points (p < 0.001), respectively.
Conclusions: Significant gains were observed in malaria QA/QC practices over the pilot. However, these advances did not translate into improved accuracy of malaria diagnostic performance perhaps because of the limited duration of the QA pilot implementation.
Keywords: Accuracy; Kenya; Laboratory; Malaria; Microscopy; Quality assurance.
Figures
References
-
- Division of Malaria Control . Kenya annual malaria report 2012/2013. Nairobi: Ministry of Public Health and Sanitation; 2013.
-
- Division of Malaria Control . National malaria strategy 2009–2017. Nairobi: Ministry of Public Health and Sanitation; 2009.
-
- WHO . Guidelines for the treatment of malaria. 2. Geneva: World Health Organization; 2010. - PubMed
-
- WHO . Malaria microscopy quality assurance manual version 1. Geneva: World Health Organization; 2009.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

