MicroRNA-146a governs fibroblast activation and joint pathology in arthritis
- PMID: 28545737
- PMCID: PMC7233286
- DOI: 10.1016/j.jaut.2017.05.006
MicroRNA-146a governs fibroblast activation and joint pathology in arthritis
Abstract
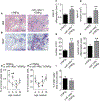
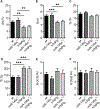
Synovial fibroblasts are key cells orchestrating the inflammatory response in arthritis. Here we demonstrate that loss of miR-146a, a key epigenetic regulator of the innate immune response, leads to increased joint destruction in a TNF-driven model of arthritis by specifically regulating the behavior of synovial fibroblasts. Absence of miR-146a in synovial fibroblasts display a highly deregulated gene expression pattern and enhanced proliferation in vitro and in vivo. Deficiency of miR-146a induces deregulation of tumor necrosis factor (TNF) receptor associated factor 6 (TRAF6) in synovial fibroblasts, leading to increased proliferation. In addition, loss of miR-146a shifts the metabolic state of fibroblasts towards glycolysis and augments the ability of synovial fibroblasts to support the generation of osteoclasts by controlling the balance of osteoclastogenic regulatory factors receptor activator of NF-κB ligand (RANKL) and osteoprotegerin (OPG). Bone marrow transplantation experiments confirmed the importance of miR-146a in the radioresistant mesenchymal compartment for the control of arthritis severity, in particular for inflammatory joint destruction. This study therefore identifies microRNA-146a as an important local epigenetic regulator of the inflammatory response in arthritis. It is a central element of an anti-inflammatory feedback loop in resident synovial fibroblasts, who are orchestrating the inflammatory response in chronic arthritis. MiR-146a restricts their activation, thereby preventing excessive tissue damage during arthritis.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest
The authors have declared that no conflict of interest exists.
Figures




References
-
- Bluml S, Redlich K, Smolen JS, Mechanisms of tissue damage in arthritis, Semin. Immunopathol 36 (5) (2014. September) 531–540. - PubMed
-
- McInnes IB, Schett G, Cytokines in the pathogenesis of rheumatoid arthritis, Nat. Rev. Immunol. 7 (6) (2007. June) 429–442. - PubMed
-
- Scott DL, Smith C, Kingsley G, Joint damage and disability in rheumatoid arthritis: an updated systematic review, Clin. Exp. Rheumatol. 21 (5 Suppl 31) (2003. Sep-Oct) S20–S27. - PubMed
-
- McInnes IB, Schett G, The pathogenesis of rheumatoid arthritis, N. Engl. J. Med. 365 (23) (2011. December 8) 2205–2219. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

