Cure modeling in real-time prediction: How much does it help?
- PMID: 28545934
- PMCID: PMC5571982
- DOI: 10.1016/j.cct.2017.05.012
Cure modeling in real-time prediction: How much does it help?
Abstract
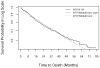
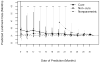
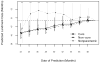
Various parametric and nonparametric modeling approaches exist for real-time prediction in time-to-event clinical trials. Recently, Chen (2016 BMC Biomedical Research Methodology 16) proposed a prediction method based on parametric cure-mixture modeling, intending to cover those situations where it appears that a non-negligible fraction of subjects is cured. In this article we apply a Weibull cure-mixture model to create predictions, demonstrating the approach in RTOG 0129, a randomized trial in head-and-neck cancer. We compare the ultimate realized data in RTOG 0129 to interim predictions from a Weibull cure-mixture model, a standard Weibull model without a cure component, and a nonparametric model based on the Bayesian bootstrap. The standard Weibull model predicted that events would occur earlier than the Weibull cure-mixture model, but the difference was unremarkable until late in the trial when evidence for a cure became clear. Nonparametric predictions often gave undefined predictions or infinite prediction intervals, particularly at early stages of the trial. Simulations suggest that cure modeling can yield better-calibrated prediction intervals when there is a cured component, or the appearance of a cured component, but at a substantial cost in the average width of the intervals.
Keywords: Bayesian bootstrap; Enrollment model; Event-based trial; Interim analysis; Weibull distribution.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures





References
-
- Bagiella E, Heitjan DF. Predicting analysis times in randomized clinical trials. Statistics in Medicine. 2001;20:2055–2063. - PubMed
-
- Ying GS, Heitjan DF, Chen TT. Nonparametric prediction of event times in randomized clinical trials. Clinical Trials. 2004;1:352–361. - PubMed
-
- Ying GS, Heitjan DF. Weibull prediction of event times in randomized clinical trials. Pharmaceutical Statistics. 2008;7:107–120. - PubMed
-
- Donovan JM, Elliott MR, Heitjan DF. Predicting event times in clinical trials when treatment arm is masked. Journal of Biopharmaceutical Statistics. 2006;16:343–356. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

