Hypoxia driven glycation: Mechanisms and therapeutic opportunities
- PMID: 28546110
- PMCID: PMC5699980
- DOI: 10.1016/j.semcancer.2017.05.008
Hypoxia driven glycation: Mechanisms and therapeutic opportunities
Abstract
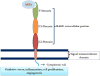
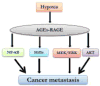
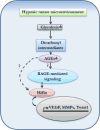
Tumor masses are deprived of oxygen and characterized by enhanced glucose uptake followed by glycolysis. Elevated glucose levels induce non-enzymatic glycosylation or glycation of proteins which leads to accumulation of advanced glycation end products (AGE). These AGE molecules bind to their respective receptors called the receptor for advanced glycation end products (RAGE) and initiate several aberrant signaling pathways leading to onset of diseases such as diabetes, Alzheimer's, atherosclerosis, heart failure and cancer. The role of AGE in cancer progression is being extensively studied in recent years. As cancer cells are hypoxic in nature and adapted to glycolysis, which induces glycation, its effects need to be understood in greater detail. Since AGE-RAGE signaling is involved in cancer progression, inhibition of AGE-RAGE interaction could be a potential therapeutic target. The purpose of this review is to highlight the role of AGE-RAGE interaction in hypoxic cancer cells.
Keywords: Advanced glycation end products; Cancer; Glycation; Hif1α; Hypoxia; RAGE.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Ousting RAGE in melanoma: A viable therapeutic target?Semin Cancer Biol. 2018 Apr;49:20-28. doi: 10.1016/j.semcancer.2017.10.008. Epub 2017 Oct 24. Semin Cancer Biol. 2018. PMID: 29079306 Free PMC article. Review.
-
AGEs, RAGEs and s-RAGE; friend or foe for cancer.Semin Cancer Biol. 2018 Apr;49:44-55. doi: 10.1016/j.semcancer.2017.07.001. Epub 2017 Jul 13. Semin Cancer Biol. 2018. PMID: 28712719 Review.
-
The anti-tumor effects of evodiamine on oral squamous cell carcinoma (OSCC) through regulating advanced glycation end products (AGE) / receptor for advanced glycation end products (RAGE) pathway.Bioengineered. 2021 Dec;12(1):5985-5995. doi: 10.1080/21655979.2021.1972082. Bioengineered. 2021. PMID: 34477479 Free PMC article.
-
Activation of the receptor for advanced glycation end products and consequences on health.Diabetes Metab Syndr. 2017 Oct-Dec;11(4):305-309. doi: 10.1016/j.dsx.2016.09.009. Epub 2016 Sep 4. Diabetes Metab Syndr. 2017. PMID: 27612394 Review.
-
Hypoxia and the Receptor for Advanced Glycation End Products (RAGE) Signaling in Cancer.Int J Mol Sci. 2021 Jul 29;22(15):8153. doi: 10.3390/ijms22158153. Int J Mol Sci. 2021. PMID: 34360919 Free PMC article. Review.
Cited by
-
Glycation Interferes with the Expression of Sialyltransferases and Leads to Increased Polysialylation in Glioblastoma Cells.Cells. 2023 Dec 2;12(23):2758. doi: 10.3390/cells12232758. Cells. 2023. PMID: 38067186 Free PMC article.
-
The Immune Tolerance Role of the HMGB1-RAGE Axis.Cells. 2021 Mar 5;10(3):564. doi: 10.3390/cells10030564. Cells. 2021. PMID: 33807604 Free PMC article. Review.
-
On the Immunometabolic Role of NF-κB in Adipocytes.Immunometabolism. 2022;4(1):e220003. doi: 10.20900/immunometab20220003. Epub 2022 Jan 29. Immunometabolism. 2022. PMID: 35251704 Free PMC article.
-
A Novel Defined Hypoxia-Related Gene Signature for Prognostic Prediction of Patients With Ewing Sarcoma.Front Genet. 2022 Jun 2;13:908113. doi: 10.3389/fgene.2022.908113. eCollection 2022. Front Genet. 2022. PMID: 35719404 Free PMC article.
-
The Taming of Nuclear Factor Erythroid-2-Related Factor-2 (Nrf2) Deglycation by Fructosamine-3-Kinase (FN3K)-Inhibitors-A Novel Strategy to Combat Cancers.Cancers (Basel). 2021 Jan 14;13(2):281. doi: 10.3390/cancers13020281. Cancers (Basel). 2021. PMID: 33466626 Free PMC article. Review.
References
-
- Malik P, Chaudhry N, Mittal R, Mukherjee TK. Role of receptor for advanced glycation end products in the complication and progression of various types of cancers. Biochimica et biophysica acta. 2015;1850(9):1898–904. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

