The Management and Imaging of Vestibular Schwannomas
- PMID: 28546250
- PMCID: PMC5690865
- DOI: 10.3174/ajnr.A5213
The Management and Imaging of Vestibular Schwannomas
Abstract
Vestibular schwannomas are the most common cerebellopontine angle tumor. During the past century, the management goals of vestibular schwannomas have shifted from total resection to functional preservation. Current treatment options include surgical resection, stereotactic radiosurgery, and observation. Imaging has become a crucial part of the initial screening, evaluation, and follow-up assessment of vestibular schwannomas. Recognizing and understanding the management objectives, various treatment modalities, expected posttreatment findings, and complications allows the radiologist to play an essential role in a multidisciplinary team by providing key findings relevant to treatment planning and outcome assessment. The authors provide a comprehensive discussion of the surgical management, role of radiation therapy and observation, imaging differential, and pre- and posttreatment imaging findings of vestibular schwannomas.
© 2017 by American Journal of Neuroradiology.
Figures



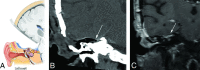
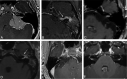
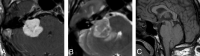
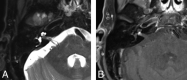


Similar articles
-
Understanding the posttreatment imaging appearance of the internal auditory canal and cerebellopontine angle.Semin Ultrasound CT MR. 2003 Jun;24(3):133-46. doi: 10.1016/s0887-2171(03)90035-7. Semin Ultrasound CT MR. 2003. PMID: 12877410 Review.
-
Keyhole retrosigmoid approach for large vestibular schwannomas: strategies to improve outcomes.Neurosurg Focus. 2018 Mar;44(3):E2. doi: 10.3171/2017.11.FOCUS17607. Neurosurg Focus. 2018. PMID: 29490546
-
[Overview of Cerebellopontine Angle Tumors].No Shinkei Geka. 2025 Jul;53(4):612-617. doi: 10.11477/mf.030126030530040612. No Shinkei Geka. 2025. PMID: 40831098 Review. Japanese.
-
Management of Vestibular Schwannomas for the Radiologist.Neuroimaging Clin N Am. 2019 Feb;29(1):173-182. doi: 10.1016/j.nic.2018.09.003. Epub 2018 Oct 31. Neuroimaging Clin N Am. 2019. PMID: 30466639 Review.
-
Susceptibility weighted imaging - a problem-solving tool in differentiation of cerebellopontine angle schwannomas and meningiomas.Neuroradiol J. 2017 Jun;30(3):253-258. doi: 10.1177/1971400916689804. Epub 2017 Jan 1. Neuroradiol J. 2017. PMID: 28627983 Free PMC article.
Cited by
-
A bibliometric and visualization analysis of global research on vestibular schwannoma.Am J Transl Res. 2023 Feb 15;15(2):755-778. eCollection 2023. Am J Transl Res. 2023. PMID: 36915774 Free PMC article.
-
Preservation of Hearing and Facial Nerve Function with the Microsurgical Excision of Large Vestibular Schwannomas: Experience with the Retrosigmoid Approach.Cureus. 2018 Dec 4;10(12):e3684. doi: 10.7759/cureus.3684. Cureus. 2018. PMID: 30761236 Free PMC article.
-
Diagnostic Value of Preoperative Electrodiagnostic Analysis in a Patient with Facial Palsy and a Large Vestibular Schwannoma: Case Report.Diagnostics (Basel). 2022 Feb 20;12(2):542. doi: 10.3390/diagnostics12020542. Diagnostics (Basel). 2022. PMID: 35204631 Free PMC article.
-
Microsurgery for Vestibular Schwannoma via Retrosigmoid Transmeatal Approach with Intraoperative Monitoring Techniques.Balkan Med J. 2021 Jul;38(4):212-221. doi: 10.5152/balkanmedj.2021.20145. Balkan Med J. 2021. PMID: 34274910 Free PMC article.
-
Adherence to Long-Term Follow-up in Patients With Sporadic Vestibular Schwannomas Managed With Serial Observation.OTO Open. 2021 Aug 9;5(3):2473974X211036653. doi: 10.1177/2473974X211036653. eCollection 2021 Jul-Sep. OTO Open. 2021. PMID: 34396030 Free PMC article.
References
-
- Mahaley MS Jr, Mettlin C, Natarajan N, et al. . Analysis of patterns of care of brain tumor patients in the United States: a study of the Brain Tumor Section of the AANS and the CNS and the Commission on Cancer of the ACS. Clin Neurosurg 1990;36:347–52 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
