Neurons within the trigeminal mesencephalic nucleus encode for the kinematic parameters of the whisker pad macrovibrissae
- PMID: 28546281
- PMCID: PMC5449554
- DOI: 10.14814/phy2.13206
Neurons within the trigeminal mesencephalic nucleus encode for the kinematic parameters of the whisker pad macrovibrissae
Abstract
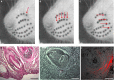
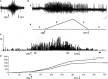
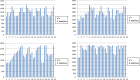
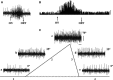
It has been recently shown in rats that spontaneous movements of whisker pad macrovibrissae elicited evoked responses in the trigeminal mesencephalic nucleus (Me5). In the present study, electrophysiological and neuroanatomical experiments were performed in anesthetized rats to evaluate whether, besides the whisker displacement per se, the Me5 neurons are also involved in encoding the kinematic properties of macrovibrissae movements, and also whether, as reported for the trigeminal ganglion, even within the Me5 nucleus exists a neuroanatomical representation of the whisker pad macrovibrissae. Extracellular electrical activity of single Me5 neurons was recorded before, during, and after mechanical deflection of the ipsilateral whisker pad macrovibrissae in different directions, and with different velocities and amplitudes. In several groups of animals, single or multiple injections of the tracer Dil were performed into the whisker pad of one side, in close proximity to the vibrissae follicles, in order to label the peripheral terminals of the Me5 neurons innervating the macrovibrissae (whisking-neurons), and therefore, the respective perikaria within the nucleus. Results showed that: (1) the whisker pad macrovibrissae were represented in the medial-caudal part of the Me5 nucleus by a single cluster of cells whose number seemed to match that of the macrovibrissae; (2) macrovibrissae mechanical deflection elicited significant responses in the Me5 whisking-neurons, which were related to the direction, amplitude, and frequency of the applied deflection. The specific functional role of Me5 neurons involved in encoding proprioceptive information arising from the macrovibrissae movements is discussed within the framework of the whole trigeminal nuclei activities.
Keywords: Me5 proprioception of macrovibrissae movements; Me5 whisking‐neurons; rat; trigeminal mesencephalic nucleus.
© 2017 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
All the authors declare that they have no current or potential conflict of interest including any financial support that could inappropriately influence their work.
Figures



Similar articles
-
The mesencephalic-hypoglossal nuclei loop as a possible central pattern generator for rhythmical whisking in rats.Exp Brain Res. 2018 Nov;236(11):2899-2911. doi: 10.1007/s00221-018-5347-7. Epub 2018 Aug 2. Exp Brain Res. 2018. PMID: 30073387
-
Evidence for a trigeminal mesencephalic-hypoglossal nuclei loop involved in controlling vibrissae movements in the rat.Exp Brain Res. 2016 Mar;234(3):753-61. doi: 10.1007/s00221-015-4503-6. Epub 2015 Dec 8. Exp Brain Res. 2016. PMID: 26645304
-
Involvement of trigeminal mesencephalic nucleus in kinetic encoding of whisker movements.Brain Res Bull. 2014 Mar;102:37-45. doi: 10.1016/j.brainresbull.2014.01.007. Epub 2014 Feb 8. Brain Res Bull. 2014. PMID: 24518654
-
Role of the trigeminal mesencephalic nucleus in rat whisker pad proprioception.Behav Brain Funct. 2010 Nov 15;6:69. doi: 10.1186/1744-9081-6-69. Behav Brain Funct. 2010. PMID: 21078134 Free PMC article.
-
Cortical control of whisker movement.Annu Rev Neurosci. 2014;37:183-203. doi: 10.1146/annurev-neuro-062012-170344. Epub 2014 May 9. Annu Rev Neurosci. 2014. PMID: 24821429 Review.
Cited by
-
Astrocyte-induced firing in primary afferent axons.iScience. 2025 Feb 12;28(3):112006. doi: 10.1016/j.isci.2025.112006. eCollection 2025 Mar 21. iScience. 2025. PMID: 40104051 Free PMC article.
-
The mesencephalic-hypoglossal nuclei loop as a possible central pattern generator for rhythmical whisking in rats.Exp Brain Res. 2018 Nov;236(11):2899-2911. doi: 10.1007/s00221-018-5347-7. Epub 2018 Aug 2. Exp Brain Res. 2018. PMID: 30073387
-
Selective Denervation of the Facial Dermato-Muscular Complex in the Rat: Experimental Model and Anatomical Basis.Front Neuroanat. 2021 Mar 22;15:650761. doi: 10.3389/fnana.2021.650761. eCollection 2021. Front Neuroanat. 2021. PMID: 33828465 Free PMC article.
-
Coding of whisker motion across the mouse face.Elife. 2019 Feb 28;8:e41535. doi: 10.7554/eLife.41535. Elife. 2019. PMID: 30816844 Free PMC article.
References
-
- Berthoud, H. R. , Kressel M., and Neuhuber W. L.. 1992. An anterograde tracing study of the vagal innervation of the rat liver, portal vein and biliary system. Anat. Embryol. 186:431–442. - PubMed
-
- Birdwell, J. A. , Solomon J. H., Thajchayapong M., Taylor M. A., Cheely M., Towal R. B., et al. 2007. Biomechanical models for radial distance determination by the rat vibrissal system. J. Neurophysiol. 89:104–117. - PubMed
-
- Brecht, M. , Preilowski B., and Merzenich M. M.. 1997. Functional architecture of the mystacial vibrissae. Behav. Brain Res. 84:81–97. - PubMed
-
- Copray, J. C. , Ter Horst G. J., Liem R. S., and van Willigen J. D.. 1990. Neurotransmitters and neuropeptides within the mesencephalic trigeminal nucleus of the rat: an immunohistochemical analysis. Neuroscience 37:399–411. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

