Dentate Gyrus Contributes to Retrieval as well as Encoding: Evidence from Context Fear Conditioning, Recall, and Extinction
- PMID: 28546308
- PMCID: PMC5490069
- DOI: 10.1523/JNEUROSCI.3029-16.2017
Dentate Gyrus Contributes to Retrieval as well as Encoding: Evidence from Context Fear Conditioning, Recall, and Extinction
Abstract
Dentate gyrus (DG) is widely thought to provide a teaching signal that enables hippocampal encoding of memories, but its role during retrieval is poorly understood. Some data and models suggest that DG plays no role in retrieval; others encourage the opposite conclusion. To resolve this controversy, we evaluated the effects of optogenetic inhibition of dorsal DG during context fear conditioning, recall, generalization, and extinction in male mice. We found that (1) inhibition during training impaired context fear acquisition; (2) inhibition during recall did not impair fear expression in the training context, unless mice had to distinguish between similar feared and neutral contexts; (3) inhibition increased generalization of fear to an unfamiliar context that was similar to a feared one and impaired fear expression in the conditioned context when it was similar to a neutral one; and (4) inhibition impaired fear extinction. These effects, as well as several seemingly contradictory published findings, could be reproduced by BACON (Bayesian Context Fear Algorithm), a physiologically realistic hippocampal model positing that acquisition and retrieval both involve coordinated activity in DG and CA3. Our findings thus suggest that DG contributes to retrieval and extinction, as well as to the initial establishment of context fear.SIGNIFICANCE STATEMENT Despite abundant evidence that the hippocampal dentate gyrus (DG) plays a critical role in memory, it remains unclear whether the role of DG relates to memory acquisition or retrieval. Using contextual fear conditioning and optogenetic inhibition, we show that DG contributes to both of these processes. Using computational simulations, we identify specific mechanisms through which the suppression of DG affects memory performance. Finally, we show that DG contributes to fear extinction learning, a process in which learned fear is attenuated through exposures to a fearful context in the absence of threat. Our data resolve a long-standing question about the role of DG in memory and provide insight into how disorders affecting DG, including aging, stress, and depression, influence cognitive processes.
Keywords: context; dentate gyrus; extinction; fear conditioning; hippocampus; memory.
Copyright © 2017 the authors 0270-6474/17/376359-13$15.00/0.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
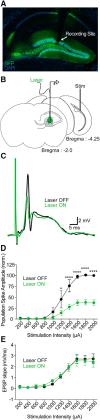
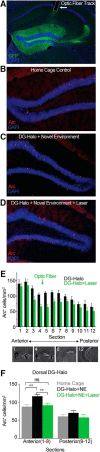
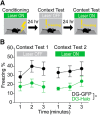
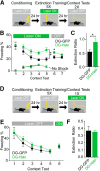

Similar articles
-
Distinct hippocampal engrams control extinction and relapse of fear memory.Nat Neurosci. 2019 May;22(5):753-761. doi: 10.1038/s41593-019-0361-z. Epub 2019 Apr 1. Nat Neurosci. 2019. PMID: 30936555 Free PMC article.
-
Extinction training suppresses activity of fear memory ensembles across the hippocampus and alters transcriptomes of fear-encoding cells.Neuropsychopharmacology. 2024 Nov;49(12):1872-1882. doi: 10.1038/s41386-024-01897-0. Epub 2024 Jun 14. Neuropsychopharmacology. 2024. PMID: 38877180
-
Disrupting nNOS-PSD-95 coupling in the hippocampal dentate gyrus promotes extinction memory retrieval.Biochem Biophys Res Commun. 2017 Nov 4;493(1):862-868. doi: 10.1016/j.bbrc.2017.09.003. Epub 2017 Sep 6. Biochem Biophys Res Commun. 2017. PMID: 28888982
-
Extinction in human fear conditioning.Biol Psychiatry. 2006 Aug 15;60(4):361-8. doi: 10.1016/j.biopsych.2005.10.006. Epub 2006 Feb 28. Biol Psychiatry. 2006. PMID: 16503330 Review.
-
Dentate gyrus circuits for encoding, retrieval and discrimination of episodic memories.Nat Rev Neurosci. 2020 Mar;21(3):153-168. doi: 10.1038/s41583-019-0260-z. Epub 2020 Feb 10. Nat Rev Neurosci. 2020. PMID: 32042144 Free PMC article. Review.
Cited by
-
Rapamycin attenuates reconsolidation of a backwards-conditioned aversive stimuli in female mice.Psychopharmacology (Berl). 2024 Mar;241(3):601-612. doi: 10.1007/s00213-024-06544-6. Epub 2024 Feb 5. Psychopharmacology (Berl). 2024. PMID: 38311691
-
Expanding the canon: An inclusive neurobiology of thalamic and subthalamic fear circuits.Neuropharmacology. 2023 Mar 15;226:109380. doi: 10.1016/j.neuropharm.2022.109380. Epub 2022 Dec 23. Neuropharmacology. 2023. PMID: 36572176 Free PMC article. Review.
-
The ontogeny of memory persistence and specificity.Dev Cogn Neurosci. 2019 Apr;36:100591. doi: 10.1016/j.dcn.2018.09.002. Epub 2018 Sep 29. Dev Cogn Neurosci. 2019. PMID: 30316637 Free PMC article. Review.
-
Propranolol can induce PTSD-like memory impairments in rats.Brain Behav. 2018 Jan 18;8(2):e00905. doi: 10.1002/brb3.905. eCollection 2018 Feb. Brain Behav. 2018. PMID: 29484264 Free PMC article.
-
Data-driven criteria to assess fear remission and phenotypic variability of extinction in rats.Philos Trans R Soc Lond B Biol Sci. 2018 Mar 19;373(1742):20170035. doi: 10.1098/rstb.2017.0035. Philos Trans R Soc Lond B Biol Sci. 2018. PMID: 29352033 Free PMC article.
References
-
- Aurnhammer C, Haase M, Muether N, Hausl M, Rauschhuber C, Huber I, Nitschko H, Busch U, Sing A, Ehrhardt A, Baiker A (2012) Universal real-time PCR for the detection and quantification of adeno-associated virus serotype 2-derived inverted terminal repeat sequences. Hum Gene Ther Methods 23:18–28. 10.1089/hgtb.2011.034 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
