AMP-Activated Protein Kinase: An Ubiquitous Signaling Pathway With Key Roles in the Cardiovascular System
- PMID: 28546359
- PMCID: PMC5447810
- DOI: 10.1161/CIRCRESAHA.117.309633
AMP-Activated Protein Kinase: An Ubiquitous Signaling Pathway With Key Roles in the Cardiovascular System
Abstract
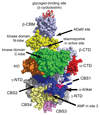
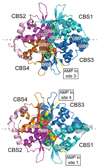
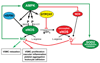
The AMP-activated protein kinase (AMPK) is a key regulator of cellular and whole-body energy homeostasis, which acts to restore energy homoeostasis whenever cellular energy charge is depleted. Over the last 2 decades, it has become apparent that AMPK regulates several other cellular functions and has specific roles in cardiovascular tissues, acting to regulate cardiac metabolism and contractile function, as well as promoting anticontractile, anti-inflammatory, and antiatherogenic actions in blood vessels. In this review, we discuss the role of AMPK in the cardiovascular system, including the molecular basis of mutations in AMPK that alter cardiac physiology and the proposed mechanisms by which AMPK regulates vascular function under physiological and pathophysiological conditions.
Keywords: AMP-activated protein kinase; heart; heart defects (congenital); metabolism; vasculature.
© 2017 American Heart Association, Inc.
Figures


References
-
- Daskalopoulos EP, Dufeys C, Beauloye C, Bertrand L, Horman S. AMPK in cardiovascular diseases. EXS. 2016;107:179–201. - PubMed
-
- Daskalopoulos EP, Dufeys C, Bertrand L, Beauloye C, Horman S. AMPK in cardiac fibrosis and repair: Actions beyond metabolic regulation. J Mol Cell Cardiol. 2016;91:188–200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

